RamiprilCAS# 87333-19-5 |
- Tolbutamide
Catalog No.:BCC5001
CAS No.:64-77-7
- Melatonin
Catalog No.:BCN2196
CAS No.:73-31-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 87333-19-5 | SDF | Download SDF |
| PubChem ID | 5362129 | Appearance | Powder |
| Formula | C23H32N2O5 | M.Wt | 416.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (240.09 mM) H2O : 1 mg/mL (2.40 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
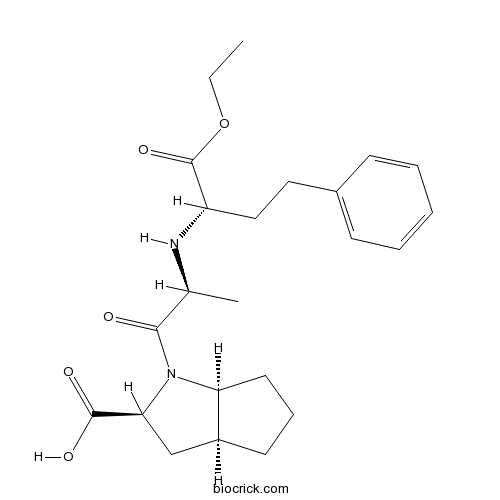
| Chemical Name | (2S,3aS,6aS)-1-[(2S)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-3,3a,4,5,6,6a-hexahydro-2H-cyclopenta[b]pyrrole-2-carboxylic acid | ||
| SMILES | CCOC(=O)C(CCC1=CC=CC=C1)NC(C)C(=O)N2C3CCCC3CC2C(=O)O | ||
| Standard InChIKey | HDACQVRGBOVJII-JBDAPHQKSA-N | ||
| Standard InChI | InChI=1S/C23H32N2O5/c1-3-30-23(29)18(13-12-16-8-5-4-6-9-16)24-15(2)21(26)25-19-11-7-10-17(19)14-20(25)22(27)28/h4-6,8-9,15,17-20,24H,3,7,10-14H2,1-2H3,(H,27,28)/t15-,17-,18-,19-,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-peptide, competitive angiotensin converting enzyme (ACE) inhibitor (IC50 = 5 nM). Prodrug which is hydrolyzed in vivo to the active metabolite ramiprilat. Displays protective effects on endothelium against high-glucose induced dysfunction. Exhibits antihypertensive effects. |

Ramipril Dilution Calculator

Ramipril Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4009 mL | 12.0045 mL | 24.009 mL | 48.0181 mL | 60.0226 mL |
| 5 mM | 0.4802 mL | 2.4009 mL | 4.8018 mL | 9.6036 mL | 12.0045 mL |
| 10 mM | 0.2401 mL | 1.2005 mL | 2.4009 mL | 4.8018 mL | 6.0023 mL |
| 50 mM | 0.048 mL | 0.2401 mL | 0.4802 mL | 0.9604 mL | 1.2005 mL |
| 100 mM | 0.024 mL | 0.12 mL | 0.2401 mL | 0.4802 mL | 0.6002 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ramipril is an angiotensin-converting enzyme (ACE) inhibitor with IC50 of 5 nM.
- AIM-100
Catalog No.:BCC1333
CAS No.:873305-35-2
- IKK-16 (IKK Inhibitor VII)
Catalog No.:BCC4555
CAS No.:873225-46-8
- Ganoderone A
Catalog No.:BCN2448
CAS No.:873061-79-1
- Ivacaftor (VX-770)
Catalog No.:BCC2478
CAS No.:873054-44-5
- 3,29-Dibenzoyl karounitriol
Catalog No.:BCN2717
CAS No.:873001-54-8
- 6-Aminouracil
Catalog No.:BCC8768
CAS No.:873-83-6
- 3-Methoxyoxohernandaline
Catalog No.:BCN8107
CAS No.:872729-34-5
- 1,2,3,10-Tetramethoxy-9-(2-hydroxy-4,5-dimethoxybenzyloxy)oxoaporphine
Catalog No.:BCN8120
CAS No.:872729-33-4
- Dabigatran etexilate mesylate
Catalog No.:BCC1511
CAS No.:872728-81-9
- MEDICA 16
Catalog No.:BCC7956
CAS No.:87272-20-6
- 7-Epi-5-eudesmene-1beta,11-diol
Catalog No.:BCN7701
CAS No.:87261-77-6
- Ro 3306
Catalog No.:BCC4007
CAS No.:872573-93-8
- 1,7-Dihydroxy-4-methoxyxanthone
Catalog No.:BCN7602
CAS No.:87339-76-2
- TC-P 262
Catalog No.:BCC6155
CAS No.:873398-67-5
- Fortuneine
Catalog No.:BCN6401
CAS No.:87340-25-8
- Triangularine
Catalog No.:BCN2051
CAS No.:87340-27-0
- PU-H71
Catalog No.:BCC1872
CAS No.:873436-91-0
- Lupeolic acid
Catalog No.:BCN6520
CAS No.:87355-32-6
- GDC-0152
Catalog No.:BCC2252
CAS No.:873652-48-3
- Demethylcarolignan E
Catalog No.:BCN7018
CAS No.:873694-46-3
- Omecamtiv mecarbil
Catalog No.:BCC3710
CAS No.:873697-71-3
- PLX647
Catalog No.:BCC6370
CAS No.:873786-09-5
- TATU
Catalog No.:BCC2822
CAS No.:873798-09-5
- BMS-599626 Hydrochloride
Catalog No.:BCC1426
CAS No.:873837-23-1
Early Treatment With Zofenopril and Ramipril in Combination With Acetyl Salicylic Acid in Patients With Left Ventricular Systolic Dysfunction After Acute Myocardial Infarction: Results of a 5-Year Follow-up of Patients of the SMILE-4 Study.[Pubmed:28195948]
J Cardiovasc Pharmacol. 2017 May;69(5):298-304.
The SMILE-4 study showed that in patients with left ventricular dysfunction (LVD) after acute myocardial infarction, early treatment with zofenopril plus acetyl salicylic acid is associated with an improved 1-year survival, free from death or hospitalization for cardiovascular (CV) causes, as compared to Ramipril plus acetyl salicylic acid. We now report CV outcomes during a 5-year follow-up of the patients of the SMILE-4 study. Three hundred eighty-six of the 518 patients completing the study (51.2%) could be tracked after the study end and 265 could be included in the analysis. During the 5.5 (+/-2.1) years of follow-up, the primary endpoint occurred in 27.8% of patients originally randomized and treated with zofenopril and in 43.8% of patients treated with Ramipril [odds ratio (OR) and 95% confidence interval, 0.65 (0.43-0.98), P = 0.041]. Such a result was achieved through a significantly larger reduction in CV hospitalization under zofenopril [OR: 0.61 (0.37-0.99), P = 0.047], whereas reduction in mortality rate with zofenopril did not achieve statistical significance versus Ramipril [OR: 0.75 (0.36-1.59), P = 0.459]. These results were in line with those achieved during the initial 1-year follow-up. Benefits of early treatment of patients with LVD after acute myocardial infarction with zofenopril are sustained over many years as compared to Ramipril.
Protective effect of losartan and ramipril against stress induced insulin resistance and related complications: Anti-inflammatory mechanisms.[Pubmed:28259714]
Eur J Pharmacol. 2017 Apr 15;801:54-61.
Chronic restraint stress (CRS) is known to cause various behavioural and biochemical alterations, leading to several negative health outcomes. The present study was designed to explore the impact of inhibiting Renin angiotensin aldosterone system (RAAS) and inflammatory pathways in stress pathophysiology. In the present study, male LACA mice were subjected to restraint stress daily for 30 days. Losartan, nimesulide, Ramipril, minocycline and their combinations were administered 45min prior to restraint stress daily and their effects were observed. Restraint stressed mice depicted depression like behavior along with increased oxidative stress markers in their brains. CRS induced insulin resistance depicted by hyperglycemia, hyperinsulinemia, hypercholesteremia, increased glycosylated hemoglobin and HOMA-IR. Besides, treatment with losartan, nimesulide, Ramipril and minocycline significantly restored the behavioural and biochemical alterations and improved insulin sensitivity in stressed mice. Combination treatments synergistically reversed depression like behavior and decreased plasma glucose levels. Moreover they restored insulin levels, glycosylated hemoglobin levels and HOMA-IR values to the normal. This study signifies the synergistic effect of simultaneously blocking RAS and inflammatory pathways in stress pathophysiology.
Comparison of endothelial function improvement estimated with reactive hyperemia index between ramipril and telmisartan in hypertensive patients.[Pubmed:28228970]
Clin Hypertens. 2017 Feb 15;23:4.
BACKGROUND: Endothelium has a function to regulate vascular tone by releasing mediators either vasodilating or vasoconstricting blood vessels. Endothelial dysfunction can be measured conveniently by Reactive Hyperemia Index (RHI) with a peripheral arterial tonometry. Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II (AT II) receptor blockers (ARBs) are considered to have beneficial effects on endothelium through inhibition of AT II. This study was performed to compare the effect of ACEIs or ARBs on endothelial function estimated by RHI in hypertensive patients. METHODS: Twenty consecutive patients with hypertension (57.9 +/- 11.3 years, 60% men) were assigned to receive treatment with Ramipril or telmisartan for eight weeks (n = 10 per group). Blood pressure (BP) and RHI were measured at baseline and after eight weeks treatment. RESULTS: The two groups were similar in terms of demographic and laboratory characteristics. But baseline systolic BP and pulse pressure (PP) were higher in telmisartan group than Ramipril group (systolic BP, 159 +/- 6.83 vs 150 +/- 7.49, p = 0.028; PP, 75.0 +/- 14.0 vs 60.3 +/- 12.4, p = 0.034). In both groups, systolic and diastolic BP decreased significantly after eight weeks treatment (p < 0.05 for each). Although PP reduced in both group (Ramipril group, 60.3 +/- 12.4 mm Hg to 50.4 +/- 7.60 mm Hg; telmisartan group, 75.0 +/- 14.0 mm Hg to 57.4 +/- 15.1 mm Hg), change was statistically remarkable only in telmisartan group. During eight weeks, there was no significant changes of RHI in both groups. There was a positive relationship between decrease of PP after 8 weeks and the improvement of endothelial function only in Ramipril group, but not in telmisartan group (Ramipril group, r = 0.671, p = 0.034; telmisartan group, r = -0.487, p = 0.153). CONCLUSIONS: Despite PP reduction effect favoring endothelial function, it's not correlated with RHI improvement with telmisartan. These findings suggest telmisartan itself may negatively influence endothelium dependent vasodilatation different from Ramipril.
The effects of alpha 1-adrenoceptor blockade and angiotensin converting enzyme inhibition on central and brachial blood pressure and vascular reactivity: the doxazosin-ramipril study.[Pubmed:27885499]
Heart Vessels. 2017 Jun;32(6):674-684.
We aimed to study whether inhibition of the renin-angiotensin-aldosterone system has effects on vascular structure and function beyond the effects on blood pressure reduction alone. Patients with mild-to-moderate hypertension (n = 61, age 54 +/- 12 years, 34% women) received the angiotensin converting enzyme inhibitor Ramipril 10 mg or the alpha 1-adrenoceptor blocker doxazosin 8 mg double-blind for 12 weeks. Aortic blood pressure, pulse wave velocity, and augmentation index were assessed by applanation tonometry. Endothelial function was studied by forearm post-ischemic flow mediated vasodilatation and by pulse wave analysis with beta 2-adrenoceptor agonist stimulation. Skin microvascular reactivity was assessed by laser Doppler fluxmetry and iontophoresis. Treatment with doxazosin or Ramipril reduced aortic and brachial blood pressures (all P < 0.001), with greater reductions in aortic than brachial systolic blood pressures (P = 0.021) and aortic/brachial pulse pressure ratio (P = 0.005). Compared to doxazosin, Ramipril reduced carotid-femoral and carotid-radial pulse wave velocity (both P < 0.05). Forearm endothelial dependent and independent vasodilatation, assessed by post-ischemic flow mediated vasodilatation and glyceryl trinitrate, and by pulse wave analysis remained unchanged by both doxazosin and Ramipril. In addition, skin microvascular endothelial dependent (acetylcholine) and independent vasodilatation (sodium nitroprusside) remained unchanged. In conclusion, Ramipril reduced indices of aortic stiffness, suggesting that angiotensin converting enzyme inhibitor therapy may have effects beyond blood pressure reduction. However, treatment did not appear to influence endothelial function. Evidence of endothelial dysfunction and its possible improvement by antihypertensive treatment might require more advanced hypertension.This study is registered at ClinicalTrials.gov (NCT02901977) and at EudraCT (# 2007-000631-25).


