Remoxipride hydrochlorideSelective D2-like antagonist CAS# 73220-03-8 |
- TAK-593
Catalog No.:BCC5142
CAS No.:1005780-62-0
- ZM323881
Catalog No.:BCC2073
CAS No.:193001-14-8
- Pazopanib (GW-786034)
Catalog No.:BCC1286
CAS No.:444731-52-6
- Motesanib
Catalog No.:BCC1776
CAS No.:453562-69-1
- Pazopanib Hydrochloride
Catalog No.:BCC3708
CAS No.:635702-64-6
- Nintedanib (BIBF 1120)
Catalog No.:BCC3661
CAS No.:656247-17-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 73220-03-8 | SDF | Download SDF |
| PubChem ID | 15565709 | Appearance | Powder |
| Formula | C16H24BrClN2O3 | M.Wt | 407.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
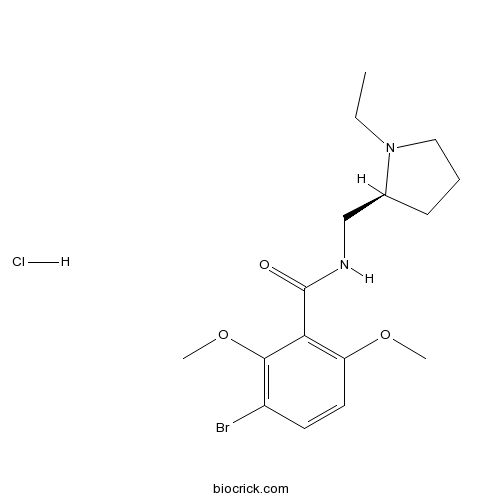
| Chemical Name | 3-bromo-N-[[(2S)-1-ethylpyrrolidin-2-yl]methyl]-2,6-dimethoxybenzamide;hydrochloride | ||
| SMILES | CCN1CCCC1CNC(=O)C2=C(C=CC(=C2OC)Br)OC.Cl | ||
| Standard InChIKey | WCPXLMIPGMFZMY-MERQFXBCSA-N | ||
| Standard InChI | InChI=1S/C16H23BrN2O3.ClH/c1-4-19-9-5-6-11(19)10-18-16(20)14-13(21-2)8-7-12(17)15(14)22-3;/h7-8,11H,4-6,9-10H2,1-3H3,(H,18,20);1H/t11-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dopamine D2 receptor antagonist showing selectivity over D3 and D4 receptors (Ki values are ~ 300, ~ 1600, and ~ 2800 nM for D2, D3 and D4 receptors respectively). Exhibits antipsychotic activity in vivo with no extrapyramidal side effects. 50-fold more potent than sulpiride in antagonising the effects of apomorphine in the rat. |

Remoxipride hydrochloride Dilution Calculator

Remoxipride hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4526 mL | 12.263 mL | 24.526 mL | 49.0521 mL | 61.3151 mL |
| 5 mM | 0.4905 mL | 2.4526 mL | 4.9052 mL | 9.8104 mL | 12.263 mL |
| 10 mM | 0.2453 mL | 1.2263 mL | 2.4526 mL | 4.9052 mL | 6.1315 mL |
| 50 mM | 0.0491 mL | 0.2453 mL | 0.4905 mL | 0.981 mL | 1.2263 mL |
| 100 mM | 0.0245 mL | 0.1226 mL | 0.2453 mL | 0.4905 mL | 0.6132 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chloranthalactone E
Catalog No.:BCN7466
CAS No.:73215-92-6
- Xamoterol hemifumarate
Catalog No.:BCC6861
CAS No.:73210-73-8
- Baptifoline
Catalog No.:BCN7988
CAS No.:732-50-3
- (d(CH2)51,Tyr(Me)2,Arg8)-Vasopressin
Catalog No.:BCC6011
CAS No.:73168-24-8
- Effusol
Catalog No.:BCN2928
CAS No.:73166-28-6
- Fenticonazole nitrate
Catalog No.:BCC8983
CAS No.:73151-29-8
- Ferruginine
Catalog No.:BCN1911
CAS No.:73069-63-3
- Arecoline
Catalog No.:BCN8537
CAS No.:73069-28-9
- Scutebarbatine D
Catalog No.:BCN8536
CAS No.:910099-76-2
- Praeruptorin D
Catalog No.:BCN4990
CAS No.:73069-28-0
- (+)-Praeruptorin A
Catalog No.:BCN4989
CAS No.:73069-27-9
- Praeruptorin A
Catalog No.:BCN4987
CAS No.:73069-25-7
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- Florfenicol
Catalog No.:BCC8984
CAS No.:73231-34-2
- Methylnaltrexone Bromide
Catalog No.:BCC1740
CAS No.:73232-52-7
- Moringin
Catalog No.:BCN7722
CAS No.:73255-40-0
- 5-O-Caffeoylshikimic acid
Catalog No.:BCN7929
CAS No.:73263-62-4
- 1,4-Dihydro-1,2-dimethyl-4-oxo-3-quinolinecarboxylic acid
Catalog No.:BCN1369
CAS No.:73281-83-1
- Deoxynojirimycin hydrochloride
Catalog No.:BCN2626
CAS No.:73285-50-4
- BAY 61-3606
Catalog No.:BCC1406
CAS No.:732983-37-8
- Methylnissolin
Catalog No.:BCN1368
CAS No.:733-40-4
- 3-Hydroxy-9,10-Dimethoxypterocarpan
Catalog No.:BCC8101
CAS No.:73340-41-7
- Macbecin I
Catalog No.:BCC7551
CAS No.:73341-72-7
- Albanin A
Catalog No.:BCN3290
CAS No.:73343-42-7
Solid state conformations and antidopaminergic effects of remoxipride hydrochloride and a closely related salicylamide, FLA 797, in relation to dopamine receptor models.[Pubmed:2945089]
Mol Pharmacol. 1986 Oct;30(4):345-51.
The X-ray structures of two new 2,6-disubstituted benzamides, i.e., Remoxipride hydrochloride monohydrate [-)-(S)-3-bromo-N-[(1-ethyl-2-pyrrolidinyl)methyl]-2,6-dimethoxybenza mide hydrochloride monohydrate) and FLA 797 [-)-(S)-3-bromo-N-[(1-ethyl-2-pyrrolidinyl)methyl]-6-methoxysalicylamide ), have been determined as well as the distribution coefficients. The difference in dopamine receptor blocking activity is discussed in terms of lipophilicity and solid state conformations of the two benzamides. The major difference between the solid state conformations lies in the orientation of the carboxamide moiety. In remoxipride the carbonyl group is oriented almost perpendicularly to the benzene ring, thus preventing the formation of a hydrogen-bonded pseudo-ring between the amide hydrogen and the methoxy group found in other types of o-methoxybenzamides. In FLA 797, however, this pseudo-ring is present in the planar conformation of the salicylamide moiety. This conformation is further stabilized by a hydrogen bond between the phenol group and the carbonyl oxygen. The side chain in remoxipride adopts an extended conformation in contrast to FLA 797, where the side chain has a folded conformation. The crystal structures are related to current topographic dopamine receptor models developed from more rigid antidopaminergic compounds. Based on these comparisons, it is suggested that benzamides having an N-ethyl-2-pyrrolidinylmethyl side chain interact with the receptor in the folded conformation. The binding affinity is thought to be further increased by the planar conformation of the salicylamide moiety present in FLA 797, which permits an efficient pi-pi stacking interaction.
In vivo effects of remoxipride and aromatic ring metabolites in the rat.[Pubmed:9400011]
J Pharmacol Exp Ther. 1997 Dec;283(3):1356-66.
The in vivo effects of remoxipride, in relation to some of its identified metabolites, were investigated in adult male Sprague-Dawley rats. The methods used included: (1) estimation of the in vivo rate of brain monoamine synthesis by measuring the accumulation of dihydroxyphenylalanine and 5-hydroxytryptophan after decarboxylase inhibition; (2) observations of spontaneous locomotor activity in a photocell-equipped open-field arena ( approximately 0. 5 m2); (3) treadmill locomotion ( approximately 4 m min-1); (4) inclined grid (60 degrees ) catalepsy test; (5) d-amphetamine-induced (1.0 mg kg-1) hyperlocomotion;(6) quinpirole-induced (0.4 mg kg-1) hypothermia. By use of one or more of these tests, the findings with remoxipride were as follows: First, remoxipride had a late onset of action (up to 3 h). Second, potency and efficacy depended on exposure to hepatic metabolism. Thus, intraperitoneal administration was more effective than the subcutaneous route, whereas virtually all biological effects were lost on intracerebroventricular administration. The ED50 values (micromol kg-1, neostriatal dihydroxyphenylalanine accumulation) for remoxipride and a range of its phenolic aromatic ring metabolites were: remoxipride (approximately 20), NCQ-344 (approximately 0.01), FLA-797 (approximately 0.1), FLA-908 (approximately 2.2), NCQ-436 (approximately 25) and NCQ-469 (approximately 30). Considering remoxipride as a nonclozapine atypical antipsychotic drug, together with the fact that remoxipride behaves as a prodrug in the laboratory studies above, further characterization of the pharmacodynamic profile of its metabolites remains a challenge.
Dopamine receptor pharmacology.[Pubmed:7940991]
Trends Pharmacol Sci. 1994 Jul;15(7):264-70.
Dopamine receptors are the primary targets in the treatment of schizophrenia, Parkinson's disease, and Huntington's chorea, and are discussed in this review by Philip Seeman and Hubert Van Tol. Improved therapy may be obtained by drugs that selectively target a particular subtype of dopamine receptor. Most antipsychotic drugs block D2 receptors in direct correlation to clinical potency, except clozapine, which prefers D4 receptors. D1 and D2 receptors can enhance each other's actions, possibly through subunits of the G proteins. In schizophrenia, the D2 and D3 receptor density is elevated by 10%, while the D4 receptor density is elevated by 600%. Therefore, D4 receptors may be a target for future antipsychotic drugs. While antipsychotics originally helped to discover dopamine receptors, the five cloned dopamine receptors are now facilitating the discovery of selective antipsychotic and antiparkinson drugs.
Binding characteristics of remoxipride and its metabolites to dopamine D2 and D3 receptors.[Pubmed:8405075]
Eur J Pharmacol. 1993 Jul 6;238(1):121-5.
The substituted benzamide, remoxipride, is a new atypical antipsychotic agent with good clinical efficacy and low extrapyramidal side-effect potential. In the present study, the in vitro receptor binding properties of remoxipride and several of its metabolites to rat striatal dopamine D2 and cloned human dopamine D2A and D3 receptors were investigated. Remoxipride bound to [3H]raclopride-labelled dopamine D2 receptors in rat striatum with an affinity (Ki) of 113 nM. The significantly lower affinities of remoxipride reported when [3H]spiperone was used as a radioligand are suggested to be due to methodological problems associated with the use of very high-affinity radioligands. Some of the phenolic metabolites of remoxipride found mainly in rat exhibited considerably higher affinities to dopamine D2 and D3 receptors than remoxipride itself. The pyrrolidone metabolites found mainly in the human had very low dopamine D2 and D3 affinities. The present in vitro results suggest that the behavioural effects of remoxipride in rats may reflect the effect of remoxipride and some of its high-affinity metabolites.


