Ridaforolimus (Deforolimus, MK-8669)MTOR inhibitor CAS# 572924-54-0 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
Number of papers citing our products

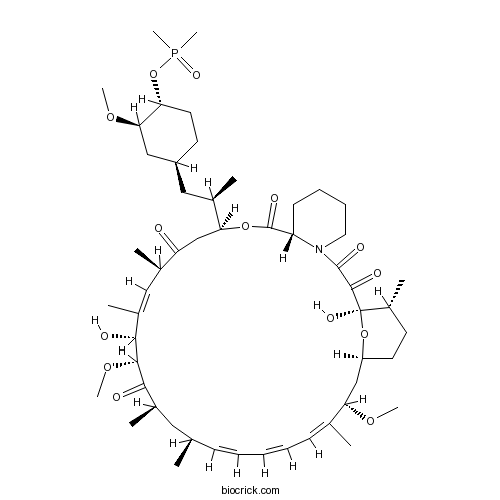
Chemical structure

3D structure
| Cas No. | 572924-54-0 | SDF | Download SDF |
| PubChem ID | 11520894 | Appearance | Powder |
| Formula | C53H84NO14P | M.Wt | 990.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AP23573; MK-8669; Ridaforolimus | ||
| Solubility | DMSO : ≥ 44 mg/mL (44.44 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (1R,9S,12S,15R,16E,18R,19R,21R,23S,24E,26E,28E,30S,32S,35R)-12-[(2R)-1-[(1S,3R,4R)-4-dimethylphosphoryloxy-3-methoxycyclohexyl]propan-2-yl]-1,18-dihydroxy-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentone | ||
| SMILES | CC1CCC2CC(C(=CC=CC=CC(CC(C(=O)C(C(C(=CC(C(=O)CC(OC(=O)C3CCCCN3C(=O)C(=O)C1(O2)O)C(C)CC4CCC(C(C4)OC)OP(=O)(C)C)C)C)O)OC)C)C)C)OC | ||
| Standard InChIKey | BUROJSBIWGDYCN-GAUTUEMISA-N | ||
| Standard InChI | InChI=1S/C53H84NO14P/c1-32-18-14-13-15-19-33(2)44(63-8)30-40-23-21-38(7)53(61,67-40)50(58)51(59)54-25-17-16-20-41(54)52(60)66-45(35(4)28-39-22-24-43(46(29-39)64-9)68-69(11,12)62)31-42(55)34(3)27-37(6)48(57)49(65-10)47(56)36(5)26-32/h13-15,18-19,27,32,34-36,38-41,43-46,48-49,57,61H,16-17,20-26,28-31H2,1-12H3/b15-13+,18-14+,33-19+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44+,45+,46-,48-,49+,53-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Deforolimus (AP23573; MK-8669) is a potent and selective mTOR inhibitor; inhibits S6 phosphorylation with an IC50 of 0.2 nM in HT-1080 cells.In Vitro:Treatment of HT-1080 fibrosarcoma cells with deforolimus results in a dose-dependent inhibition of phosphorylation of both S6 and 4E-BP1, with IC50s of 0.2 and 5.6 nM, respectively, and EC50s of 0.2 and 1.0 nM, respectively. In HT-1080 cells, the EC50 for inhibition of cell proliferation (0.5 nM) is similar to the EC50s for inhibition of S6 and 4E-BP1 phosphorylation. Exposure to deforolimus reduces the proliferation of cell lines representing a variety of tumor types. Administration of deforolimus to tumor cells in vitro elicit dose-dependent inhibition of mTOR activity with concomitant effects on cell growth and division. Deforolimus exhibits a predominantly cytostatic mode of action, consistent with the findings for other mTOR inhibitors. Potent inhibitory effects on vascular endothelial growth factor secretion, endothelial cell growth, and glucose metabolism[1].In Vivo:Deforolimus inhibits tumor growth in mice bearing PC-3 (prostate), HCT-116 (colon), MCF7 (breast), PANC-1 (pancreas), or A549 (lung) xenografts. Deforolimus inhibits tumor growth in a dose-dependent manner, with 0.3 mg/kg being the lowest dose that inhibits tumor growth significantly and 3 and 10 mg/kg doses achieving maximum inhibition[1]. References: | |||||

Ridaforolimus (Deforolimus, MK-8669) Dilution Calculator

Ridaforolimus (Deforolimus, MK-8669) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0099 mL | 5.0495 mL | 10.099 mL | 20.1979 mL | 25.2474 mL |
| 5 mM | 0.202 mL | 1.0099 mL | 2.0198 mL | 4.0396 mL | 5.0495 mL |
| 10 mM | 0.101 mL | 0.5049 mL | 1.0099 mL | 2.0198 mL | 2.5247 mL |
| 50 mM | 0.0202 mL | 0.101 mL | 0.202 mL | 0.404 mL | 0.5049 mL |
| 100 mM | 0.0101 mL | 0.0505 mL | 0.101 mL | 0.202 mL | 0.2525 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ridaforolimus (Deforolimus, MK-8669), a novel rapamycin analogue, is a novel, potent and selective inhibitor of mTOR with IC50 value of 0.2nM [1].
HT-1080 fibrosarcoma cells treated with ridaforolimus have been demonstrated to dose-dependently inhibit S6 and 4E-BP1 phosphorylation with IC50 values of 0.2 and 5.6 nM, respectively, and EC50 values of 0.2 and 1.0 nM, respectively. The antiproliferative activity of ridaforolimus has been observed in a broad panel of cell lines including the colon cancer cells HCT-116, leiomyosarcoma cells SK-UT-1, etc. Ridaforolimus has shown to block the production of VEGF production dose-dependently, with an EC50 value of 0.1nM [1].
In vivo, mice bearing MCF7 (breast), PC-3 (prostate), A549 (lung), HCT-116 (colon) or PANC-1 (pancreas) xenografts have revealed the antitumor efficacy of ridaforolimus [1].
References:
[1] Rivera VM1, Squillace RM, Miller D, Berk L, Wardwell SD, Ning Y, Pollock R, Narasimhan NI, Iuliucci JD, Wang F, Clackson T.Ridaforolimus (AP23573; MK-8669), a potent mTOR inhibitor, has broad antitumor activity and can be optimally administered using intermittent dosing regimens. Mol Cancer Ther. 2011 Jun;10(6):1059-71.
- Boc-D-Phe(4-F)-OH
Catalog No.:BCC3218
CAS No.:57292-45-2
- Boc-D-Phe(4-Cl)-OH
Catalog No.:BCC3176
CAS No.:57292-44-1
- Calmidazolium chloride
Catalog No.:BCC7410
CAS No.:57265-65-3
- Setiptiline
Catalog No.:BCC1945
CAS No.:57262-94-9
- Salsolinol-1-carboxylic acid
Catalog No.:BCC6731
CAS No.:57256-34-5
- 7-Ethoxyresorufin
Catalog No.:BCC6476
CAS No.:5725-91-7
- Methoxyresorufin
Catalog No.:BCC6296
CAS No.:5725-89-3
- Pamidronate Disodium
Catalog No.:BCC1193
CAS No.:57248-88-1
- H-Phe(3-CN)-OH
Catalog No.:BCC3182
CAS No.:57213-48-6
- Testosterone decanoate
Catalog No.:BCC9168
CAS No.:5721-91-5
- Ayanin
Catalog No.:BCN4056
CAS No.:572-32-7
- Engeletin
Catalog No.:BCN5772
CAS No.:572-31-6
- Boehmenan
Catalog No.:BCN5773
CAS No.:57296-22-7
- Liriodendrin
Catalog No.:BCN5774
CAS No.:573-44-4
- Congo Red
Catalog No.:BCC8023
CAS No.:573-58-0
- Tacalcitol
Catalog No.:BCC1975
CAS No.:57333-96-7
- Dihydroepistephamiersine 6-acetate
Catalog No.:BCN5775
CAS No.:57361-74-7
- Bombinakinin-GAP
Catalog No.:BCC5903
CAS No.:573671-91-7
- Isochlorogenic acid C
Catalog No.:BCN2498
CAS No.:57378-72-0
- Irsogladine
Catalog No.:BCC4562
CAS No.:57381-26-7
- Oroxin A
Catalog No.:BCN1202
CAS No.:57396-78-8
- Benzoin ethyl ether
Catalog No.:BCC8855
CAS No.:574-09-4
- Isoflavone
Catalog No.:BCN8508
CAS No.:574-12-9
- Fraxetin
Catalog No.:BCN5903
CAS No.:574-84-5


