SMANT hydrochlorideInhibits Smoothened (Smo) accumulation CAS# 1177600-74-6 |
- BV6
Catalog No.:BCC5372
CAS No.:1001600-56-1
- LCL161
Catalog No.:BCC1691
CAS No.:1005342-46-0
- Birinapant (TL32711)
Catalog No.:BCC2250
CAS No.:1260251-31-7
- Embelin
Catalog No.:BCN2678
CAS No.:550-24-3
- GDC-0152
Catalog No.:BCC2252
CAS No.:873652-48-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1177600-74-6 | SDF | Download SDF |
| PubChem ID | 2900213 | Appearance | Powder |
| Formula | C16H24BrClN2O | M.Wt | 375.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in water and to 100 mM in DMSO | ||
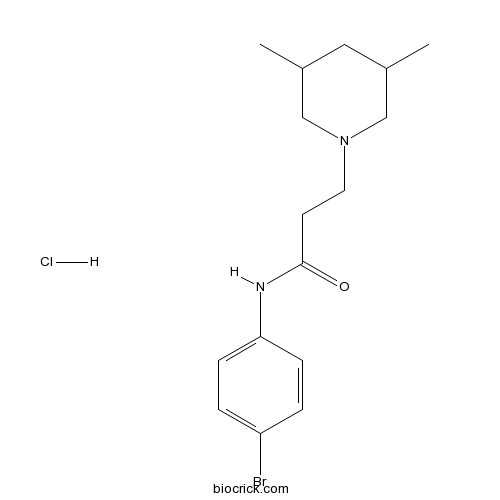
| Chemical Name | N-(4-bromophenyl)-3-(3,5-dimethylpiperidin-1-yl)propanamide;hydrochloride | ||
| SMILES | CC1CC(CN(C1)CCC(=O)NC2=CC=C(C=C2)Br)C.Cl | ||
| Standard InChIKey | XQESCHFXROUCOQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H23BrN2O.ClH/c1-12-9-13(2)11-19(10-12)8-7-16(20)18-15-5-3-14(17)4-6-15;/h3-6,12-13H,7-11H2,1-2H3,(H,18,20);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of Smoothened (Smo) signaling via a unique mechanism. Inhibits Shh-induced accumulation of Smo::EGFP fusion protein in the primary cilium (IC50 = 1.1 μM). Inhibits both wild-type Smo and an oncogenic form (SmoM2) with similar efficacy. |

SMANT hydrochloride Dilution Calculator

SMANT hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6615 mL | 13.3074 mL | 26.6149 mL | 53.2297 mL | 66.5371 mL |
| 5 mM | 0.5323 mL | 2.6615 mL | 5.323 mL | 10.6459 mL | 13.3074 mL |
| 10 mM | 0.2661 mL | 1.3307 mL | 2.6615 mL | 5.323 mL | 6.6537 mL |
| 50 mM | 0.0532 mL | 0.2661 mL | 0.5323 mL | 1.0646 mL | 1.3307 mL |
| 100 mM | 0.0266 mL | 0.1331 mL | 0.2661 mL | 0.5323 mL | 0.6654 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N20C hydrochloride
Catalog No.:BCC7292
CAS No.:1177583-87-7
- Forsythoside I
Catalog No.:BCN6430
CAS No.:1177581-50-8
- Felbamate hydrate
Catalog No.:BCC4160
CAS No.:1177501-39-1
- (R)-(+)-Blebbistatin
Catalog No.:BCC7195
CAS No.:1177356-70-5
- CKI 7 dihydrochloride
Catalog No.:BCC5614
CAS No.:1177141-67-1
- Doramectin
Catalog No.:BCC1536
CAS No.:117704-25-3
- Dexamethasone acetate
Catalog No.:BCC4775
CAS No.:1177-87-3
- Laxogenin
Catalog No.:BCN8434
CAS No.:1177-71-5
- DL-Syringaresinol
Catalog No.:BCN6053
CAS No.:1177-14-6
- LY 255283
Catalog No.:BCC7290
CAS No.:117690-79-6
- Agomelatine hydrochloride
Catalog No.:BCC4210
CAS No.:1176316-99-6
- 3-Cyano-7-ethoxycoumarin
Catalog No.:BCC7979
CAS No.:117620-77-6
- CGH 2466 dihydrochloride
Catalog No.:BCC7338
CAS No.:1177618-54-0
- Azithromycin Dihydrate
Catalog No.:BCC4631
CAS No.:117772-70-0
- Decumbenine C
Catalog No.:BCC8314
CAS No.:117772-89-1
- Desmethyl-YM 298198
Catalog No.:BCC7365
CAS No.:1177767-57-5
- AP-III-a4
Catalog No.:BCC5292
CAS No.:1177827-73-4
- NSC 23766
Catalog No.:BCC1149
CAS No.:1177865-17-6
- 3,3',4',5,6,7,8-heptamethoxyflavone
Catalog No.:BCN8203
CAS No.:1178-24-1
- Enterostatin
Catalog No.:BCC6050
CAS No.:117830-79-2
- Ac-Asp(OtBu)-OH
Catalog No.:BCC2880
CAS No.:117833-18-8
- Loreclezole hydrochloride
Catalog No.:BCC7009
CAS No.:117857-45-1
- L-CCG-l
Catalog No.:BCC6609
CAS No.:117857-93-9
- L-CCG-lll
Catalog No.:BCC6608
CAS No.:117857-95-1
Unusual 4-arsonoanilinium cationic species in the hydrochloride salt of (4-aminophenyl)arsonic acid and formed in the reaction of the acid with copper(II) sulfate, copper(II) chloride and cadmium chloride.[Pubmed:28378716]
Acta Crystallogr C Struct Chem. 2017 Apr 1;73(Pt 4):325-330.
Structures having the unusual protonated 4-arsonoanilinium species, namely in the hydrochloride salt, C6H9AsNO3(+).Cl(-), (I), and the complex salts formed from the reaction of (4-aminophenyl)arsonic acid (p-arsanilic acid) with copper(II) sulfate, i.e. hexaaquacopper(II) bis(4-arsonoanilinium) disulfate dihydrate, (C6H9AsNO3)2[Cu(H2O)6](SO4)2.2H2O, (II), with copper(II) chloride, i.e. poly[bis(4-arsonoanilinium) [tetra-mu-chlorido-cuprate(II)]], {(C6H9AsNO3)2[CuCl4]}n, (III), and with cadmium chloride, i.e. poly[bis(4-arsonoanilinium) [tetra-mu-chlorido-cadmate(II)]], {(C6H9AsNO3)2[CdCl4]}n, (IV), have been determined. In (II), the two 4-arsonoanilinium cations are accompanied by [Cu(H2O)6](2+) cations with sulfate anions. In the isotypic complex salts (III) and (IV), they act as counter-cations to the {[CuCl4](2-)}n or {[CdCl4](2-)}n anionic polymer sheets, respectively. In (II), the [Cu(H2O)6](2+) ion sits on a crystallographic centre of symmetry and displays a slightly distorted octahedral coordination geometry. The asymmetric unit for (II) contains, in addition to half the [Cu(H2O)6](2+) ion, one 4-arsonoanilinium cation, a sulfate dianion and a solvent water molecule. Extensive O-H...O and N-H...O hydrogen bonds link all the species, giving an overall three-dimensional structure. In (III), four of the chloride ligands are related by inversion [Cu-Cl = 2.2826 (8) and 2.2990 (9) A], with the other two sites of the tetragonally distorted octahedral CuCl6 unit occupied by symmetry-generated Cl-atom donors [Cu-Cl = 2.9833 (9) A], forming a two-dimensional coordination polymer network substructure lying parallel to (001). In the crystal, the polymer layers are linked across [001] by a number of bridging hydrogen bonds involving N-H...Cl interactions from head-to-head-linked As-O-H...O 4-arsonoanilinium cations. A three-dimensional network structure is formed. Cd(II) compound (IV) is isotypic with Cu(II) complex (III), but with the central CdCl6 complex repeat unit having a more regular M-Cl bond-length range [2.5232 (12)-2.6931 (10) A] compared to that in (III). This series of compounds represents the first reported crystal structures having the protonated 4-arsonoanilinium species.
Lens opacities in children using methylphenidate hydrochloride.[Pubmed:28376677]
Cutan Ocul Toxicol. 2017 Dec;36(4):362-365.
PURPOSE: To assess clinical findings of eye examination in children having attention deficit hyperactivity disorder (ADHD) administered with methylphenidate hydrochloride. METHODS: Fifty-seven consecutive patients diagnosed of ADHD and administered with oral methylphenidate hydrochloride treatment for at least one year were involved in this study (Group 1). Sixty healthy subjects (Group 2) having demographic features similar to group 1 were involved as a control group. All patients underwent detailed ophthalmological examination. RESULTS: One hundred and seventeen consecutive subjects with a mean age of 11.2 +/- 2.4 years (7-18 years) were enrolled. Fifty-seven consecutive patient (32 males, 25 females) under oral methylphenidate hydrochloride treatment (Group 1) and 60 healthy control subjects (30 males, 30 females) (Group 2)) were recruited for this prospective study. The mean methylphenidate hydrochloride dosage was 0.9 +/- 0.1 mg/kg/day and the mean duration of methylphenidate hydrochloride usage was for 2.73 +/- 0.73 years (1-7 years). High intraocular pressure was not observed in any of the patients in our study. We detected lens opacities in five eyes of five patients in group 1 (p = 0.019). The patient with the highest degree of cataract formation had been using MPH for 84 months and this patient's cataract score was P4. CONCLUSION: Long-term use of methylphenidate may cause lens opacities. In particular, patients who have been using methylphenidate for more than two years should go for regular eye examination.
Biophysical Study on the Interaction between Eperisone Hydrochloride and Human Serum Albumin Using Spectroscopic, Calorimetric, and Molecular Docking Analyses.[Pubmed:28380300]
Mol Pharm. 2017 May 1;14(5):1656-1665.
Eperisone hydrochloride (EH) is widely used as a muscle relaxant for patients with muscular contracture, low back pain, or spasticity. Human serum albumin (HSA) is a highly soluble negatively charged, endogenous and abundant plasma protein ascribed with the ligand binding and transport properties. The current study was undertaken to explore the interaction between EH and the serum transport protein, HSA. Study of the interaction between HSA and EH was carried by UV-vis, fluorescence quenching, circular dichroism (CD), Fourier transform infrared (FTIR) spectroscopy, Forster's resonance energy transfer, isothermal titration calorimetry and differential scanning calorimetry. Tryptophan fluorescence intensity of HSA was strongly quenched by EH. The binding constants (Kb) were obtained by fluorescence quenching, and results show that the HSA-EH interaction revealed a static mode of quenching with binding constant Kb approximately 10(4) reflecting high affinity of EH for HSA. The negative DeltaG degrees value for binding indicated that HSA-EH interaction was a spontaneous process. Thermodynamic analysis shows HSA-EH complex formation occurs primarily due to hydrophobic interactions, and hydrogen bonds were facilitated at the binding of EH. EH binding induces alpha-helix of HSA as obtained by far-UV CD and FTIR spectroscopy. In addition, the distance between EH (acceptor) and Trp residue of HSA (donor) was calculated 2.18 nm using Forster's resonance energy transfer theory. Furthermore, molecular docking results revealed EH binds with HSA, and binding site was positioned in Sudlow Site I of HSA (subdomain IIA). This work provides a useful experimental strategy for studying the interaction of myorelaxant with HSA, helping to understand the activity and mechanism of drug binding.
Fabrication yields of serially harvested calf-fed Holstein steers fed zilpaterol hydrochloride.[Pubmed:28380524]
J Anim Sci. 2017 Mar;95(3):1209-1218.
Holstein steers ( = 110) were fed zilpaterol hydrochloride (ZH) for 0 or 20 d before slaughter during a 280-d serial harvest study. Cattle were harvested every 28 d beginning at 254 d on feed (DOF) and concluding at 534 DOF. After slaughter, carcasses were chilled for 48 h and then fabricated into boneless closely trimmed or denuded subprimals, lean trim, trimmable fat, and bone. Inclusion of ZH increased cold side weight (CSW) by 10.3 kg ( < 0.01; 212.7 vs. 202.4 kg [SEM 1.96]) and saleable yield by 10.4 kg ( < 0.01; 131.9 vs. 121.5 kg [SEM 1.16]) in calf-fed Holstein steer carcasses. Additionally, saleable yield as a percentage of CSW increased (
Selective identification of hedgehog pathway antagonists by direct analysis of smoothened ciliary translocation.[Pubmed:22554036]
ACS Chem Biol. 2012 Jun 15;7(6):1040-8.
Hedgehog (Hh) signaling promotes tumorigenesis. The accumulation of the membrane protein Smoothened (Smo) within the primary cilium (PC) is a key event in Hh signal transduction, and many pharmacological inhibitors identified to date target Smo's actions. Smo ciliary translocation is inhibited by some pathway antagonists, while others promote ciliary accumulation, an outcome that can lead to a hypersensitive state on renewal of Hh signaling. To identify novel inhibitory compounds acting on the critical mechanistic transition of Smo accumulation, we established a high content screen to directly analyze Smo ciliary translocation. Screening thousands of compounds from annotated libraries of approved drugs and other agents, we identified several new classes of compounds that block Sonic hedgehog-driven Smo localization within the PC. Selective analysis was conducted on two classes of Smo antagonists. One of these, DY131, appears to inhibit Smo signaling through a common binding site shared by previously reported Smo agonists and antagonists. Antagonism by this class of compound is competed by high doses of Smo-binding agonists such as SAG and impaired by a mutation that generates a ligand-independent, oncogenic form of Smo (SmoM2). In contrast, a second antagonist of Smo accumulation within the PC, SMANT, was less sensitive to SAG-mediated competition and inhibited SmoM2 at concentrations similar to those that inhibit wild-type Smo. Our observations identify important differences among Hh antagonists and the potential for development of novel therapeutic approaches against mutant forms of Smo that are resistant to current therapeutic strategies.


