Sarafotoxin S6bEndothelin receptor agonist; non-selective CAS# 116303-65-2 |
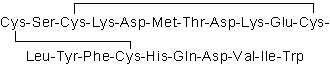
- A 419259 trihydrochloride
Catalog No.:BCC4308
CAS No.:1435934-25-0
- PP 2 (AG 1879)
Catalog No.:BCC3631
CAS No.:172889-27-9
- PD173955
Catalog No.:BCC3999
CAS No.:260415-63-2
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- WH-4-023
Catalog No.:BCC8051
CAS No.:837422-57-8
- KX2-391
Catalog No.:BCC5080
CAS No.:897016-82-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure
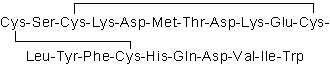
3D structure
| Cas No. | 116303-65-2 | SDF | Download SDF |
| PubChem ID | 16132420 | Appearance | Powder |
| Formula | C110H159N27O34S5 | M.Wt | 2564 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in 20% acetonitrile | ||
| Sequence | CSCKDMTDKECLYFCHQDVIW (Modifications: Disulfide bridge between: 1-15, 3-11) | ||
| SMILES | CCC(C)C(C(=O)NC(C(C)C)C(=O)NC(CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)C(CC(=O)O)NC(=O)C(CCC(=O)O)NC(=O)C(CC3=CN=CN3)NC(=O)C(CS)NC(=O)C(CC4=CC=CC=C4)NC(=O)C(CC5=CC=C(C=C5)O)NC(=O)C(CC(C)C)NC(=O)C(CS)NC(=O)C(CCC(=O)O)NC(=O)C(CCCCN)NC(=O)C(CC(=O)O)NC(=O)C(C(C)O)NC(=O)C(CCSC)NC(=O)C(CC(=O)O)NC(=O)C(CCCCN)NC(=O)C(CS)NC(=O)C(CO)NC(=O)C(CS)N | ||
| Standard InChIKey | GNKMHJZCKAUKSE-RAALVGGVSA-N | ||
| Standard InChI | InChI=1S/C110H162N26O35S5/c1-9-55(6)88(108(168)134-87(54(4)5)107(167)129-77(110(170)171)40-59-45-115-64-22-14-13-21-62(59)64)135-102(162)76(44-86(148)149)127-93(153)67(29-31-82(140)141)120-99(159)73(41-60-46-114-52-116-60)125-106(166)81(51-175)132-98(158)71(38-57-19-11-10-12-20-57)124-97(157)72(39-58-25-27-61(139)28-26-58)123-96(156)70(37-53(2)3)122-105(165)80(50-174)131-94(154)68(30-32-83(142)143)119-91(151)65(23-15-17-34-111)117-101(161)75(43-85(146)147)128-109(169)89(56(7)138)136-95(155)69(33-36-176-8)121-100(160)74(42-84(144)145)126-92(152)66(24-16-18-35-112)118-104(164)79(49-173)133-103(163)78(47-137)130-90(150)63(113)48-172/h10-14,19-22,25-28,45-46,52-56,63,65-81,87-89,115,137-139,172-175H,9,15-18,23-24,29-44,47-51,111-113H2,1-8H3,(H,114,116)(H,117,161)(H,118,164)(H,119,151)(H,120,159)(H,121,160)(H,122,165)(H,123,156)(H,124,157)(H,125,166)(H,126,152)(H,127,153)(H,128,169)(H,129,167)(H,130,150)(H,131,154)(H,132,158)(H,133,163)(H,134,168)(H,135,162)(H,136,155)(H,140,141)(H,142,143)(H,144,145)(H,146,147)(H,148,149)(H,170,171)/t55-,56+,63-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,87-,88-,89-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vasoconstrictor peptide; non-selective endothelin receptor agonist. |

Sarafotoxin S6b Dilution Calculator

Sarafotoxin S6b Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Clemizole hydrochloride
Catalog No.:BCC1486
CAS No.:1163-36-6
- 6-Aldehydoisoophiopogonanone A
Catalog No.:BCN2860
CAS No.:116291-82-8
- Pyrroside B
Catalog No.:BCN4042
CAS No.:116271-35-3
- MCB-613
Catalog No.:BCC3982
CAS No.:1162656-22-5
- Levobetaxolol HCl
Catalog No.:BCC4671
CAS No.:116209-55-3
- Aflatoxin B1
Catalog No.:BCC9212
CAS No.:1162-65-8
- Complanatoside
Catalog No.:BCN8213
CAS No.:116183-66-5
- Alexine
Catalog No.:BCN2054
CAS No.:116174-63-1
- Brevicolline
Catalog No.:BCN2459
CAS No.:20069-02-7
- VU 0361737
Catalog No.:BCC4596
CAS No.:1161205-04-4
- G-15
Catalog No.:BCC6058
CAS No.:1161002-05-6
- Phenamil
Catalog No.:BCC7673
CAS No.:1161-94-0
- SKF 86002 dihydrochloride
Catalog No.:BCC7236
CAS No.:116339-68-5
- Fumonisin B1
Catalog No.:BCC2461
CAS No.:116355-83-0
- Acetylexidonin
Catalog No.:BCN3279
CAS No.:116368-90-2
- 2alpha-hydroxy-3beta-acetyloxy-betulic acid
Catalog No.:BCN3072
CAS No.:1163728-89-9
- Loureirin C
Catalog No.:BCN3761
CAS No.:116384-24-8
- 3',4',7-Trimethoxyflavan
Catalog No.:BCN6042
CAS No.:116384-26-0
- Z-Tyr-OH
Catalog No.:BCC2747
CAS No.:1164-16-5
- Androstanolone acetate
Catalog No.:BCC8826
CAS No.:1164-91-6
- SR 3576
Catalog No.:BCC7999
CAS No.:1164153-22-3
- 9S-10alpha-Hydroxyepigambogic acid
Catalog No.:BCN3080
CAS No.:1164201-85-7
- Fargesone A
Catalog No.:BCN6417
CAS No.:116424-69-2
- Fargesone B
Catalog No.:BCN6415
CAS No.:116424-70-5
The chimeric peptide [Lys(-2)-Arg(-1)]-sarafotoxin-S6b, composed of the endothelin pro-sequence and sarafotoxin, retains the salt-bridge staple between Arg(-1) and Asp8 previously observed in [Lys(-2)-Arg(-1)]-endothelin. Implications of this salt-bridge in the contractile activity and the oxidative folding reaction.[Pubmed:10583392]
Eur J Biochem. 1999 Dec;266(3):977-85.
The chimeric peptide [Lys(-2)-Arg(-1)]-sarafotoxin-S6b (KR-SRTb) designed from the Lys-2-Arg-1 dipeptide of the endothelin pro-sequence and the sarafotoxin-S6b sequence was synthesized. Its contractile activity was found to be decreased markedly when compared with that of the parent SRTb. In contrast, the extension by the Lys-Arg dipeptide was found to increase the formation of the native disulfide isomer (82/18 versus 96/4) when the reaction was carried out in the presence of redox reagents. The solution structure of KR-SRTb was determined by NMR as a function of pH. In the carboxylic acid state, the structure consists of the cystine-stabilized alpha-helical motif, with the alpha-helical part spanning residues 9-15, and of an unstructured C-terminal tail. In the carboxylate state, the structure is characterized by a salt-bridge between Arg(-1) and Asp8, which we identified previously in the [Lys(-2)-Arg(-1)]-endothelin-1 peptide (KR-ET-1). The fact that this salt-bridge is commonly observed in KR-SRTb and KR-ET-1, despite the 33% sequence difference between the corresponding parental peptides, highlights the remarkable adaptability of the Lys-Arg extension for the formation of a special salt-bridge. As a consequence, this salt-bridge, which does not depend on either the 4-7 sequence of the loop or the C-terminal sequence, appears to be particularly well suited to improve the stability of the cystine-stabilized alpha-helical motif. Therefore, because of its high yield in the native disulfide arrangement and its high permissiveness for sequence mutation in the 4-7 loop, such a stabilized cystine-stabilized alpha-helical motif could be a valuable scaffold for the presentation of a library of constrained short peptides.
Comparison of the contractile effects and binding kinetics of endothelin-1 and sarafotoxin S6b in rat isolated renal artery.[Pubmed:9154335]
Br J Pharmacol. 1997 May;121(2):253-63.
1. To date, only two mammalian endothelin (ET) receptors, termed ETA and ETB, have been cloned, sequenced and characterized. However, several functional studies of isolated blood vessels suggest that ET-1-induced contractions may be mediated by multiple ETA receptors. In this study, the ETA receptors in renal arteries isolated from Wistar rats were characterized by isometric tension recording and radioligand binding techniques. 2. ET-1, Sarafotoxin S6b (StxS6b) and ET-3 produced concentration-dependent contraction with similar response maxima in endothelium-denuded arteries, whereas the ETB receptor-selective agonist StxS6c was inactive. ET-1 and StxS6b were equipotent and 30 times more potent than ET-3. This agonist profile, together with the findings that the ETA receptor-selective antagonists, BQ-123 and FR-139317 caused concentration-dependent, rightward shifts of the concentration-effect curves to each agonist indicated that ET-1-induced contractions in rat renal artery were mediated via ETA receptors. 3. BQ-123 and FR-139317 were both significantly more potent inhibitors of contractions induced by StxS6b or ET-3 than of responses to ET-1, raising the possibility that a component of ET-1-induced contraction was mediated through atypical, BQ-123 (or FR-139317)-insensitive ETA receptors. However, in competition binding studies, specific [125I]-ET-1 and [125I]-StxS6b binding to rat renal artery sections was completely abolished by BQ-123 in a manner consistent with an action at a single site. Thus, competition binding studies did not provide any supportive evidence of the existence of a BQ-123-insensitive ETA receptor. 4. Additional studies revealed marked differences in the kinetics of [125I]-ET-1 and [125I]-StxS6b binding. Following a 3 h period of association of [125I]-ET-1 with its receptors, no significant dissociation of receptor-bound [125I]-ET-1 was observed during a 4 h washout period. In stark contrast, dissociation studies revealed that specific [125I]-StxS6b binding to ETA receptors was reversible (t0.5diss, 100 min). A series of association binding studies were also consistent with the specific binding of [125I]-ET-1 and [125I]-StxS6b being irreversible and reversible processes, respectively. 5. Thus, differences in BQ-123 potency against ET-1 and StxS6b-induced contractions in rat renal arteries might be due to differences in the kinetics of agonist binding, rather than due to the existence of atypical ETA receptors.
Histamine release from Weibel-Palade bodies of toad aortas induced by endothelin-1 and sarafotoxin-S6b.[Pubmed:7573984]
Anat Rec. 1995 Jul;242(3):374-82.
BACKGROUND: Endothelin-1 (ET-1) and sarafotoxin-S6b (STX) induce a remarkable degranulation of Weibel-Palade (WP) bodies prior to the vasocontraction of toad aortas. As WP bodies play the role of a reservoir site of the histamine in the endothelial cells, there is the possibility that ET-1 and STX evoke the release of histamine from WP bodies of this vessel. METHODS: Histamine concentrations were assayed by high-performance liquid chromatography (HPLC) from the perfusate after being perfused with a solution containing ET-1 and STX. Each vessel was fixed and embedded for conventional electron microscopy and immunoelectron microscopy using antihistamine sera. RESULTS: The appreciable concentrations of histamine were assayed by HPLC from the perfusate after the toad aortas were perfused with a solution containing ET-1 and STX. The immunoelectron microscopy revealed that histamine immunoreactive gold particles in the WP bodies remarkably decreased in number in the treated samples when compared to the control ones. Our immunoelectron micrographs indicated that the release of histamine from the endothelial cells occurred in association with the degranulation and the exocytosis of the WP bodies after treatment with ET-1 and STX. CONCLUSIONS: The present study clearly shows that ET-1 and STX induce the histamine release from WP bodies of the toad aortas by means of HPLC and immunoelectron microscopy. Histamine discharged from the WP bodies may be involved in the vasocontraction evoked by ET-1 and STX.
Atypical receptors mediate the response to endothelin-1 and sarafotoxin S6b in the human umbilical artery.[Pubmed:9874167]
Eur J Pharmacol. 1998 Dec 4;362(2-3):167-72.
The receptors mediating smooth muscle response to endothelin-1 and Sarafotoxin S6b in the human umbilical artery were investigated in vitro. Both agonists induced contractions that were unaffected by the endothelin ET(B) receptor antagonist BQ 788 (10(-9), 10(-8), 10(-7) M). The non-selective endothelin ET(A/B) receptor antagonist PD 142893 (10(-7) M) decreased the contraction induced by endothelin-1. PD 142893 (10(-9) M) enhanced the contraction induced by Sarafotoxin S6b whereas higher concentrations had no effect. Removing the endothelium did not affect the antagonising action of PD 142893 on endothelin-1-induced contractions while the enhancement of the Sarafotoxin S6b-induced contraction was abolished. Sarafotoxin S6b induced relaxation in segments precontracted by 5-hydroxytryptamine and exposed to the endothelin ET(A) receptor antagonist BQ 123 (10(-7) M) and PD 142893 (10(-9) M) abolished this relaxation. These endothelial receptors seem neither to be classical endothelin ET(A) nor endothelin ET(B) receptors and they are not activated by endothelin-1.
Different endothelin receptors involved in endothelin-1- and sarafotoxin S6B-induced contractions of the human isolated coronary artery.[Pubmed:7889304]
Br J Pharmacol. 1994 Dec;113(4):1471-9.
1. Endothelin receptors, that mediate contraction of the human isolated coronary artery, were characterized by use of a number of agonists and antagonists. Contraction induced by the non-selective agonists, endothelin (ET)-1 and Sarafotoxin S6b, was compared in endothelium-intact and endothelium-denuded ring segments. The effects of ET-1 and BQ-123 (an ETA receptor antagonist) were investigated both in ring segments and in spirally cut strips. Lastly, the effect of phosphoramidon was studied on contraction induced by big-ET-1. 2. The order of agonist potency (pD2) in endothelium-intact coronary artery ring segments was: ET-1 (8.27) approximately Sarafotoxin S6b (8.16) > big-ET-1 (< 7.1) approximately ET-3 (< 6.9). [Ala1,3,11,15]ET-1 (ETB receptor agonist) caused significant contraction only at 1 microM, whereas 0.3 microM big-ET-3 had no effect. Removal of the endothelium in ring segments did not affect the contractile response to ET-1 or to Sarafotoxin S6b. 3. After a full concentration-response curve had been obtained to ET-1 or Sarafotoxin S6b, further contractions of the endothelium-intact coronary artery segments could only be achieved by applying ET-1 in segments exposed to Sarafotoxin S6b, and not the reverse. 4. BQ-123 (0.1 microM) antagonized contractions of endothelium-intact ring segments induced by Sarafotoxin S6b (pKB 7.86). Only 10 microM BQ-123 antagonized contractions induced by ET-1 (pKB 5.75). FR139317 was also more potent against Sarafotoxin S6b (pKB 8.24-8.47) than against ET-1 (pKB 6.11). [Ala1,3,11,15]ET-1 (1 microM) had no effect on the contractile response to ET-1 or to Sarafotoxin S6b. 5. In strip preparations with intact endothelium, the pD2 of ET-l increased to 9.04 =/- 0.16 (vs.8.50 +/- 0.07 in rings), and BQ-123 (1 microM) caused a rightward shift of the ET-l induced concentration response curve (pKB 6.62 vs. 5.75 in rings).6. Contractile responses to big-ET-1 of endothelium-intact coronary artery segments were attenuated in the presence of phosphoramidon (100 microM), indicating conversion of big-ET-1 to ET-1 within the coronary artery segment.7. The present study indicates that ET-1 and Sarafotoxin S6b contract the human isolated coronary artery via different receptors, which can probably be best characterized as subtypes of the ETA receptor.Furthermore, it is demonstrated that the type of preparation (ring or strip) may affect the potency of ET-1 as an agonist and of BQ-123 as an antagonist.
Studies on the disulfide bridges of sarafotoxins. Chemical synthesis of sarafotoxin S6B and its homologue with different disulfide bridges.[Pubmed:2080919]
Biochem Int. 1990 Sep;21(6):1051-7.
Sarafotoxin S6b, a strong vasocontractile peptide with 21 amino acid residues containing two sets of disulfide bridges, was chemically synthesized. The retention time on a reversed-phase HPLC, lethal and vasocontractile activities of natural Sarafotoxin S6b agree with those of the synthetic [Cys1-15, Cys3-11]-Sarafotoxin S6b. The combination of the disulfide bridges of Sarafotoxin S6b is the same as that of endothelin-1, a mammalian vasocontractile peptide, which shows a high degree of sequence homology and shares several common pharmacological properties with sarafotoxins.


