TAK-438Blocker of potassium-competitive acid CAS# 1260141-27-2 |

- ML-7 hydrochloride
Catalog No.:BCC1770
CAS No.:110448-33-4
- Istaroxime
Catalog No.:BCC1660
CAS No.:203737-93-3
- Dynasore
Catalog No.:BCC1088
CAS No.:304448-55-3
- Istaroxime hydrochloride
Catalog No.:BCC1661
CAS No.:374559-48-5
- 20-HETE
Catalog No.:BCC1301
CAS No.:79551-86-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure
3D structure
| Cas No. | 1260141-27-2 | SDF | Download SDF |
| PubChem ID | 45375887 | Appearance | Powder |
| Formula | C21H20FN3O6S | M.Wt | 461.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Vonoprazan Fumarate | ||
| Solubility | Soluble in DMSO > 10 mM | ||
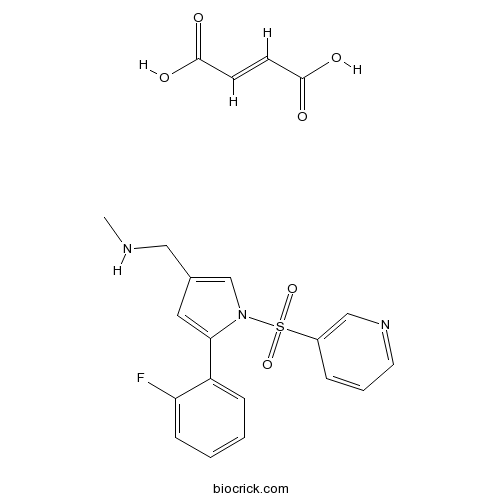
| Chemical Name | (E)-but-2-enedioic acid;1-[5-(2-fluorophenyl)-1-pyridin-3-ylsulfonylpyrrol-3-yl]-N-methylmethanamine | ||
| SMILES | CNCC1=CN(C(=C1)C2=CC=CC=C2F)S(=O)(=O)C3=CN=CC=C3.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | ROGSHYHKHPCCJW-WLHGVMLRSA-N | ||
| Standard InChI | InChI=1S/C17H16FN3O2S.C4H4O4/c1-19-10-13-9-17(15-6-2-3-7-16(15)18)21(12-13)24(22,23)14-5-4-8-20-11-14;5-3(6)1-2-4(7)8/h2-9,11-12,19H,10H2,1H3;1-2H,(H,5,6)(H,7,8)/b;2-1+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | TAK-438 is a potassium-competitive acid blocker (P-CAB) of H,K-ATPase with IC50 value of 19 nM. | |||||
| Targets | H,K-ATPase | |||||
| IC50 | 19 nM | |||||

TAK-438 Dilution Calculator

TAK-438 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.167 mL | 10.8352 mL | 21.6704 mL | 43.3407 mL | 54.1759 mL |
| 5 mM | 0.4334 mL | 2.167 mL | 4.3341 mL | 8.6681 mL | 10.8352 mL |
| 10 mM | 0.2167 mL | 1.0835 mL | 2.167 mL | 4.3341 mL | 5.4176 mL |
| 50 mM | 0.0433 mL | 0.2167 mL | 0.4334 mL | 0.8668 mL | 1.0835 mL |
| 100 mM | 0.0217 mL | 0.1084 mL | 0.2167 mL | 0.4334 mL | 0.5418 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
TAK-438 is a potassium-competitive acid blocker (P-CAB) that reversibly inhibits gastric H+, K+-ATPase, [1] [2] with ID50 values of 0.86 mg/kg to histamine-stimulated acid secretion in anesthetized rats [1].
Gastric H+, K+-ATPase has a key role in the final secretion step of gastric acid, transporting H+, via an electroneutral exchange of H+ for K+, into the secretory canaliculus in parietal cells [1].
In cultured gastric glands, TAK-438 treatment resulted in a longer and stronger acid formation inhibition. The inhibition effect of TAK-438 on acid secretion seemed to be associated with gastric parietal cell physiology. After cultured gastric glands were incubated with TAK-438 for 2 h and hence the incubation buffer was replaced with the CK buffer, the acid formation stimulated by forskolin slowly recovered, but the acid formation was inhibited immediately in a concentration-dependent manner [2].
In rats, 1-4, 5-8, and 9-12 h after the administration of TAK-438 at 3 mg/kg p.o., acid secretion stimulated by histamine was strongly inhibited. 24 to 27 h after administration of TAK-438, there was an inhibition rate of 40%, and this was a significant and sustained inhibition. In Heidenhain pouch dogs treated with doses of 0.1 to 1 mg/kg TAK-438 p.o., the acid secretion stimulated by histamine was inhibited dose-dependently, and this effect lasted for > 48 h. 1, 3, and 6 h after administration of 1 mg/kg TAK-438 completely inhibited the acid secretion stimulated by histamine [1].
References:
[1]. Yasunobu Hori, Jun Matsukawa, Toshiyuki Takeuchi, et al. A Study Comparing the Antisecretory Effect of TAK-438, a Novel Potassium-Competitive Acid Blocker, with Lansoprazole in Animals. Journal of Pharmacology and Experimental Therapeutics, 2011, 337:797-804.
[2]. Jun Matsukawa, Yasunobu Hori, Haruyuki Nishida, et al. A comparative study on the modes of action of TAK-438, a novel potassium-competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochemical Pharmacology, 2011, 81:1145-1151.
- 28-Deoxonimbolide
Catalog No.:BCN4717
CAS No.:126005-94-5
- Carminic acid
Catalog No.:BCN6541
CAS No.:1260-17-9
- Phlegmanol C
Catalog No.:BCN6138
CAS No.:1260-05-5
- Polygalic acid
Catalog No.:BCN3172
CAS No.:1260-04-4
- Oxethazaine
Catalog No.:BCC3832
CAS No.:126-27-2
- Sarsasapogenin
Catalog No.:BCN1269
CAS No.:126-19-2
- Solasodine
Catalog No.:BCN2346
CAS No.:126-17-0
- Griseofulvin
Catalog No.:BCC5327
CAS No.:126-07-8
- 10-O-Ethylcannabitriol
Catalog No.:BCN7312
CAS No.:1259515-25-7
- SCH 23390 hydrochloride
Catalog No.:BCC6849
CAS No.:125941-87-9
- VU 0155041 sodium salt
Catalog No.:BCC7642
CAS No.:1259372-69-4
- JP 1302 dihydrochloride
Catalog No.:BCC7449
CAS No.:1259314-65-2
- 3-Oxo-21alpha-methoxy-24,25,26,27-tetranortirucall-7-ene-23(21)-lactone
Catalog No.:BCN7028
CAS No.:1260173-73-6
- TCS 21311
Catalog No.:BCC2443
CAS No.:1260181-14-3
- Birinapant (TL32711)
Catalog No.:BCC2250
CAS No.:1260251-31-7
- 3-O-beta-Allopyranosyl-(1->4)-beta-oleandropyranosyl-11-O-isobutyryl-12-O-acetyltenacigenin B
Catalog No.:BCN6765
CAS No.:1260252-18-3
- GSK 525768A
Catalog No.:BCC1603
CAS No.:1260530-25-3
- Atractyloside A
Catalog No.:BCN5383
CAS No.:126054-77-1
- 6-O-Methylcerevisterol
Catalog No.:BCN6139
CAS No.:126060-09-1
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Rubiyunnanin C
Catalog No.:BCN8045
CAS No.:1261030-04-9
- 11-Oxo-mogroside V
Catalog No.:BCN2509
CAS No.:126105-11-1
- Siamenoside I
Catalog No.:BCN2540
CAS No.:126105-12-2
- GNF179
Catalog No.:BCC5175
CAS No.:1261114-01-5
Characteristics of the Novel Potassium-Competitive Acid Blocker Vonoprazan Fumarate (TAK-438).[Pubmed:27287852]
Adv Ther. 2016 Jul;33(7):1140-57.
UNLABELLED: Proton pump inhibitors (PPIs) are widely prescribed as first-line therapy for the treatment of acid-related diseases, such as peptic ulcers and gastro-esophageal reflux disease, and for the eradication of Helicobacter pylori. However, the therapeutic efficacy of conventional PPIs is considered limited because: (1) they are unstable under acidic conditions and require an enteric-coated formulation in clinical use; (2) they show high interindividual variability in pharmacokinetics due to genetic polymorphisms of cytochrome P450 (CYP) 2C19 metabolism; (3) they have a relatively slow onset of pharmacological action and may require several doses to achieve optimal acid suppression and symptom relief; and (4) they often do not provide stable suppression of gastric acid secretion over 24 h. Vonoprazan fumarate (TAK-438, hereinafter referred to as "vonoprazan") is a new potassium-competitive acid blocker (P-CAB) developed to resolve the above limitations of conventional PPIs. Various physicochemical data have shown that vonoprazan has a high solubility and stability over a broad pH range in aqueous conditions. In addition, vonoprazan has a more potent and longer-lasting acid suppression effect than the conventional PPI, lansoprazole. Preclinical pharmacokinetic studies have shown that vonoprazan is accumulated and retained in the stomach for more than 24 h, even after it is eliminated from the plasma. From these findings, we propose that vonoprazan, which possesses a novel mode of action, can improve on the outcomes seen with conventional PPI-based treatments for acid-related diseases. FUNDING: This review project, including the publication of this article, was funded by Takeda Pharmaceutical Company Limited.
Disposition and metabolism of TAK-438 (vonoprazan fumarate), a novel potassium-competitive acid blocker, in rats and dogs.[Pubmed:27225050]
Xenobiotica. 2017 Mar;47(3):255-266.
1. Following oral administration of [(14)C]TAK-438, the radioactivity was rapidly absorbed in rats and dogs. The apparent absorption of the radioactivity was high in both species. 2. After oral administration of [(14)C]TAK-438 to rats, the radioactivity in most tissues reached the maximum at 1-hour post-dose. By 168-hour post-dose, the concentrations of the radioactivity were at very low levels in nearly all the tissues. In addition, TAK-438F was the major component in the stomach, whereas TAK-438F was the minor component in the plasma and other tissues. High accumulation of TAK-438F in the stomach was observed after oral and intravenous administration. 3. TAK-438F was a minor component in the plasma and excreta in both species. Its oxidative metabolite (M-I) and the glucuronide of a secondary metabolite formed by non-oxidative metabolism of M-I (M-II-G) were the major components in the rat and dog plasma, respectively. The glucuronide of M-I (M-I-G) and M-II-G were the major components in the rat bile and dog urine, respectively, and most components in feces were other unidentified metabolites. 4. The administered radioactive dose was almost completely recovered. The major route of excretion of the drug-derived radioactivity was via the feces in rats and urine in dogs.
In vitro metabolism of TAK-438, vonoprazan fumarate, a novel potassium-competitive acid blocker.[Pubmed:27414183]
Xenobiotica. 2017 Dec;47(12):1027-1034.
1. TAK-438, vonoprazan fumarate, is a novel orally active potassium-competitive acid blocker, developed as an antisecretory drug. In this study, we investigated the in vitro metabolism of (14)C-labeled TAK-438. In human hepatocytes, M-I, M-II, M-III and M-IV-Sul were mainly formed, and these were also detected in clinical studies. N-demethylated TAK-438 was also formed as an in vitro specific metabolite. Furthermore, CYP3A4 mainly contributed to the metabolism of TAK-438 to M-I, M-III, and N-demethylated TAK-438, and CYP2B6, CYP2C19 and CYP2D6 partly catalyzed the metabolism of TAK-438. The sulfate conjugation by SULT2A1 also contributed to the metabolism of TAK-438 to form TAK-438 N-sulfate, and CYP2C9 mediated the formation of M-IV-Sul from TAK-438 N-sulfate. The metabolite M-IV, which could be another possible intermediate in the formation of M-IV-Sul, was not observed as a primary metabolite of TAK-438 in any of the in vitro studies. 2. In conclusion, TAK-438 was primarily metabolized by multiple metabolizing enzymes including CYP3A4, CYP2B6, CYP2C19, CYP2D6, and a non-CYP enzyme SULT2A1, and the influence of the CYP2C19 genotype status on gastric acid suppression post TAK-438 dosing could be small. The multiple metabolic pathways could also minimize the effects of co-administrated CYP inhibitors or inducers on the pharmacokinetics of TAK-438.


