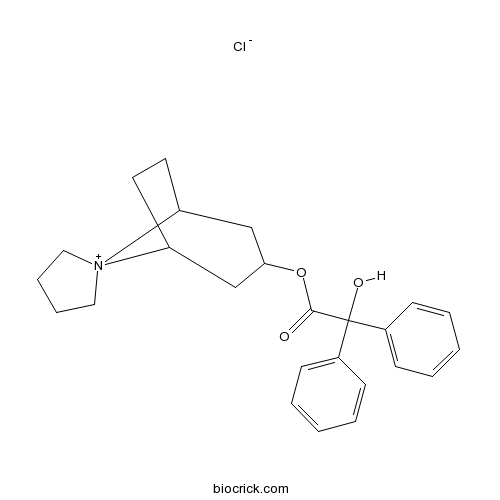
Trospium chlorideAntimuscarinic agent CAS# 10405-02-4 |
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- Balaglitazone
Catalog No.:BCC1395
CAS No.:199113-98-9
- Inolitazone
Catalog No.:BCC1652
CAS No.:223132-37-4
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- Aleglitazar
Catalog No.:BCC1337
CAS No.:475479-34-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 10405-02-4 | SDF | Download SDF |
| PubChem ID | 107979 | Appearance | Powder |
| Formula | C25H30ClNO3 | M.Wt | 427.96 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 100 mg/mL (233.67 mM) DMSO : 33.33 mg/mL (77.88 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | spiro[8-azoniabicyclo[3.2.1]octane-8,1'-azolidin-1-ium]-3-yl 2-hydroxy-2,2-diphenylacetate;chloride | ||
| SMILES | C1CC[N+]2(C1)C3CCC2CC(C3)OC(=O)C(C4=CC=CC=C4)(C5=CC=CC=C5)O.[Cl-] | ||
| Standard InChIKey | RVCSYOQWLPPAOA-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C25H30NO3.ClH/c27-24(25(28,19-9-3-1-4-10-19)20-11-5-2-6-12-20)29-23-17-21-13-14-22(18-23)26(21)15-7-8-16-26;/h1-6,9-12,21-23,28H,7-8,13-18H2;1H/q+1;/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Trospium Chloride is a competitive muscarinic cholinergic receptor antagonist.
Target: mAChR
Trospium chloride is an antimuscarinic agent indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency. Trospium has pharmacologic properties that are distinct from other antimuscarinic agents [1]. After oral administration, absorption of the hydrophilic trospium chloride is slow and incomplete. Peak plasma concentrations (Cmax) of approximately 4 ng/mL are reached 4-5 hours after administration of a 20 mg immediate-release preparation. The mean bioavailability is approximately 10% and decreases by concomitant food intake (to a mean of 26% of the fasting area under the plasma concentration-time curve [AUC]). Trospium chloride displays dose proportional increases in AUC and Cmax after a single dose within the clinically relevant dose range (20-60 mg). The mean volume of distribution is approximately 350-800 L [2]. References: | |||||

Trospium chloride Dilution Calculator

Trospium chloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3367 mL | 11.6833 mL | 23.3667 mL | 46.7333 mL | 58.4167 mL |
| 5 mM | 0.4673 mL | 2.3367 mL | 4.6733 mL | 9.3467 mL | 11.6833 mL |
| 10 mM | 0.2337 mL | 1.1683 mL | 2.3367 mL | 4.6733 mL | 5.8417 mL |
| 50 mM | 0.0467 mL | 0.2337 mL | 0.4673 mL | 0.9347 mL | 1.1683 mL |
| 100 mM | 0.0234 mL | 0.1168 mL | 0.2337 mL | 0.4673 mL | 0.5842 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Trospium chloride is an antimuscarinic agent indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency. Trospium has pharmacologic properties that are distinct from other antimuscarinic ag
- 8alpha-Methacryloyloxybalchanin
Catalog No.:BCN4756
CAS No.:104021-39-8
- Tussilagone
Catalog No.:BCN2770
CAS No.:104012-37-5
- Cinnamaldehyde
Catalog No.:BCN6241
CAS No.:104-55-2
- Cinnamyl alcohol
Catalog No.:BCN4967
CAS No.:104-54-1
- Anethole
Catalog No.:BCN5373
CAS No.:104-46-1
- 4-Methoxyphenylacetic acid
Catalog No.:BCN8467
CAS No.:104-01-8
- 4'-Hydroxypiptocarphin A
Catalog No.:BCN7113
CAS No.:103994-39-4
- 13-Hydroxygermacrone
Catalog No.:BCN3556
CAS No.:103994-29-2
- Ceftiofur hydrochloride
Catalog No.:BCC8911
CAS No.:103980-44-5
- Esculentic acid
Catalog No.:BCN5856
CAS No.:103974-74-9
- 15-Nor-14-oxolabda-8(17),12-dien-18-oic acid
Catalog No.:BCN1637
CAS No.:1039673-32-9
- Oleanolic acid 3-O-beta-D-glucosyl-(1->3)-alpha-L-rhamnosyl(1->2)-alpha-L-arabinoside
Catalog No.:BCN8132
CAS No.:103956-33-8
- LP533401 hcl
Catalog No.:BCC6377
CAS No.:1040526-12-2
- Quinovic acid 3-O-alpha-L-rhamnopyranoside
Catalog No.:BCN1636
CAS No.:104055-76-7
- Dihydroobovatin
Catalog No.:BCN3982
CAS No.:104055-79-0
- 3',5'-Diprenylgenistein
Catalog No.:BCN3572
CAS No.:104055-80-3
- Rehmapicroside
Catalog No.:BCN2884
CAS No.:104056-82-8
- Cyclo(Ile-Val)
Catalog No.:BCN2410
CAS No.:104068-43-1
- Atipamezole hydrochloride
Catalog No.:BCC7521
CAS No.:104075-48-1
- Zolantidine dimaleate
Catalog No.:BCC6922
CAS No.:104076-39-3
- Fmoc-D-Glu(OtBu)-OH
Catalog No.:BCC3496
CAS No.:104091-08-9
- SR 95531 hydrobromide
Catalog No.:BCC6997
CAS No.:104104-50-9
- Kadsuric acid 3-methylester
Catalog No.:BCN3186
CAS No.:1041070-16-9
- Phellodendrine chloride
Catalog No.:BCN5934
CAS No.:104112-82-5
Randomized, double-blind, placebo-controlled trial to compare solifenacin versus trospium chloride in the relief of double-J stent-related symptoms.[Pubmed:28050642]
World J Urol. 2017 Aug;35(8):1261-1268.
PURPOSE: We aimed to compare the safety and efficacy of solifenacin versus Trospium chloride and compare each drug versus placebo regarding the relief of stent-related symptoms following uncomplicated ureteroscopic lithotripsy (URSL). METHODS: In a prospective, randomized, double-blind study, 210 eligible patients who underwent URSL with double-J stent insertion were recruited and randomly assigned to either the first group, receiving solifenacin (10 mg), second group, receiving Trospium chloride (60 mg), or the third group, receiving placebo (one tablet). All patients were kept on study medication once daily during the entire 2-week postoperative period. All subjects were asked to complete a brief-form questionnaire to assess the lower urinary symptoms, stent-related body pain and hematuria, preoperatively and 2 weeks postoperatively. RESULTS: There were no statistically significant differences among the study groups in terms of mean age, gender, anthropometric measurements, stone and stent criteria. The overall symptom score, urgency, urge incontinence, flank pain, urethral pain and gross hematuria scores were significantly lower in solifenacin group compared to Trospium chloride and placebo groups (p < 0.001). Concerning frequency and nocturia, there was no significant difference in mean scores across all groups. Drug-related side effects, particularly constipation, were higher in trospium group than in solifenacin one. CONCLUSIONS: Solifenacin treatment showed significant improvement in almost all domains of stent-related symptoms than trospium. In terms of safety and tolerance, both drugs were comparable. Future studies should be designed to address the impact of combined drugs and lower doses in the management of DJ stent-related symptoms.
[High doses of trospium chloride in patients with idiopathic overactive bladder. Data of large-scale, multicenter observational program Resource].[Pubmed:28247723]
Urologiia. 2016 Aug;(4):29-34.
PURPOSE: Evaluation of the efficacy and safety of different doses of Trospium chloride in patients with idiopathic overactive bladder. MATERIALS AND METHODS: Large-scale observational program "Resource" included 669 patients with idiopathic OAB - 359 women and 310 men. At the first visit, all patients were assigned to use of Trospium chloride at a standard dose of 45 mg per day. The results of treatment were evaluated during follow-up visits at 3, 6, 9 and 12 weeks. Depending on the results of examination, the dose was reduced in the presence of adverse events and increased in case of insufficient treatment effects. RESULTS: After 12 weeks, 102 patients have been receiving the drug at a dose of 30 mg/day, 241 - at a dose of 45 mg/day, 257 - at a dose of 60 mg/day, and 22 - at a dose of 75 mg/day. CONCLUSIONS: Individual approach to the selection of doses of Trospium chloride in patients with idiopathic OAB can be quite effective and safe measure to achieve optimal clinical outcome with a good safety profile.
Trospium chloride is absorbed from two intestinal "absorption windows" with different permeability in healthy subjects.[Pubmed:27765726]
Int J Pharm. 2016 Dec 30;515(1-2):367-373.
Intestinal P-glycoprotein is regio-selectively expressed and is a high affinity, low capacity efflux carrier for the cationic, poorly permeable trospium. Organic cation transporter 1 (OCT1) provides lower affinity but higher capacity for trospium uptake. To evaluate regional intestinal permeability, absorption profiles after gastric infusion of Trospium chloride (30mg/250ml=[I]2) for 6h and after swallowing 30mg immediate-release tablets in fasted and fed healthy subjects, were evaluated using an inverse Gaussian density function to model input rate and mean absorption time (MAT). Trospium chloride was slowly absorbed (MAT approximately 10h) after gastric infusion involving two processes with different input rates, peaking at about 3h and 7h. Input rates and MAT were influenced by dosage form and meal. In conclusion, trospium is absorbed from two "windows" located in the jejunum and cecum/ascending colon, whose uptake capacity might result from local abundance and functional interplay of P-glycoprotein and OCT1.
Effect of Trospium Chloride on Cognitive Function in Women Aged 50 and Older: A Randomized Trial.[Pubmed:28067745]
Female Pelvic Med Reconstr Surg. 2017 Mar/Apr;23(2):118-123.
OBJECTIVES: This study aimed to investigate the effect of Trospium chloride on cognitive function in postmenopausal women treated for overactive bladder (OAB). METHODS: Randomized double-blind placebo-controlled trial conducted from April 2013 to April 2015. Women aged 50 years or older seeking treatment for OAB were randomized to either Trospium chloride XR 60 mg daily or placebo. Baseline cognitive function was assessed via Hopkins Verbal Learning Test-Revised (HVLT-R), Mini Mental Status Exam, Mini Mental Status X, Digit Span, Trails A, Trails B, and Epworth Sleepiness Scale. Cognitive function was reassessed at week 1 and week 4. A priori power analysis determined that 21 subjects were needed per group. RESULTS: Although 59 women were enrolled and randomized (28 trospium and 31 placebo), 45 completed assessment (21 trospium and 24 placebo). Mean age was 68 years, 78% were white, and 44% had previously taken OAB medication. For the primary outcome, there was no difference in HVLT-R total score between trospium and placebo groups at week 4 (P = 0.29). There were also no differences based on the other cognitive tests. There was a correlation between age and the following week-4 tests: HVLT-R total score (r = -0.3, P = 0.02), HVLT-R total recall subscale (r = -0.4, P = 0.007), Trails A (r = 0.4, P = 0.002), and Trails B (r = 0.4, P = 0.004). A linear regression model found that HVLT-R total score decreased by 0.372 points for each increased year of age. CONCLUSIONS: In women aged 50 years and older, there were no changes in cognitive function between those taking trospium and placebo. Cognitive function was correlated with age.


