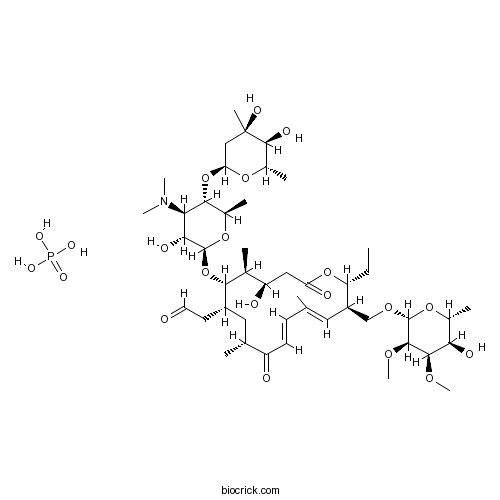
Tylosin phosphateCAS# 1405-53-4 |
- ABT-199
Catalog No.:BCC3614
CAS No.:1257044-40-8
- WEHI-539
Catalog No.:BCC2055
CAS No.:1431866-33-9
- HA14-1
Catalog No.:BCC3593
CAS No.:65673-63-4
- Obatoclax mesylate (GX15-070)
Catalog No.:BCC2234
CAS No.:803712-79-0
- ABT-263 (Navitoclax)
Catalog No.:BCC1272
CAS No.:923564-51-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1405-53-4 | SDF | Download SDF |
| PubChem ID | 6440844 | Appearance | Powder |
| Formula | C46H80NO21P | M.Wt | 1014.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | 2-[(4R,5S,6S,7R,9R,11E,13E,15R,16R)-6-[(2R,3R,4R,5S,6R)-5-[(2S,4R,5S,6S)-4,5-dihydroxy-4,6-dimethyloxan-2-yl]oxy-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-16-ethyl-4-hydroxy-15-[[(2R,3R,4R,5R,6R)-5-hydroxy-3,4-dimethoxy-6-methyloxan-2-yl]oxymethyl]-5,9,13-trimethyl-2,10-dioxo-1-oxacyclohexadeca-11,13-dien-7-yl]acetaldehyde;phosphoric acid | ||
| SMILES | CCC1C(C=C(C=CC(=O)C(CC(C(C(C(CC(=O)O1)O)C)OC2C(C(C(C(O2)C)OC3CC(C(C(O3)C)O)(C)O)N(C)C)O)CC=O)C)C)COC4C(C(C(C(O4)C)O)OC)OC.OP(=O)(O)O | ||
| Standard InChIKey | NBOODGNJLRRJNA-IAGPQMRQSA-N | ||
| Standard InChI | InChI=1S/C46H77NO17.H3O4P/c1-13-33-30(22-58-45-42(57-12)41(56-11)37(52)26(5)60-45)18-23(2)14-15-31(49)24(3)19-29(16-17-48)39(25(4)32(50)20-34(51)62-33)64-44-38(53)36(47(9)10)40(27(6)61-44)63-35-21-46(8,55)43(54)28(7)59-35;1-5(2,3)4/h14-15,17-18,24-30,32-33,35-45,50,52-55H,13,16,19-22H2,1-12H3;(H3,1,2,3,4)/b15-14+,23-18+;/t24-,25+,26-,27-,28+,29+,30-,32-,33-,35+,36-,37-,38-,39-,40-,41-,42-,43+,44+,45-,46-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tylosin phosphate Dilution Calculator

Tylosin phosphate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.9861 mL | 4.9305 mL | 9.861 mL | 19.7219 mL | 24.6524 mL |
| 5 mM | 0.1972 mL | 0.9861 mL | 1.9722 mL | 3.9444 mL | 4.9305 mL |
| 10 mM | 0.0986 mL | 0.493 mL | 0.9861 mL | 1.9722 mL | 2.4652 mL |
| 50 mM | 0.0197 mL | 0.0986 mL | 0.1972 mL | 0.3944 mL | 0.493 mL |
| 100 mM | 0.0099 mL | 0.0493 mL | 0.0986 mL | 0.1972 mL | 0.2465 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tylosin phosphate(Fradizine; Tylocine; Tylosin A) is a broad spectrum antibiotic against Gram-positive organisms and a limited range of Gram-negative organisms.
References:
[1]. Pilcher CM, et al. Impact of tylosin phosphate and distillers dried grains with solubles on energy and nutrient digestibility and flow through the gastrointestinal tract in growing pigs. J Anim Sci. 2013 Dec;91(12):5687-95.
[2]. Entorf M, et al. Tylosin susceptibility of staphylococci from bovine mastitis. Vet Microbiol. 2014 Jul 16;171(3-4):368-73.
- Gentamycin Sulfate
Catalog No.:BCC1203
CAS No.:1405-41-0
- Capreomycin Sulfate
Catalog No.:BCC4644
CAS No.:1405-37-4
- Neomycin sulfate
Catalog No.:BCC4682
CAS No.:1405-10-3
- Olopatadine HCl
Catalog No.:BCC4545
CAS No.:140462-76-6
- Heteroclitin B
Catalog No.:BCN3745
CAS No.:140461-47-8
- Heteroclitin C
Catalog No.:BCN3632
CAS No.:140460-42-0
- Ergosterol peroxide glucoside
Catalog No.:BCN6222
CAS No.:140447-22-9
- 11-Hydroxyjasmonic acid
Catalog No.:BCN6221
CAS No.:140447-14-9
- GSK 2830371
Catalog No.:BCC4179
CAS No.:1404456-53-6
- ML 281
Catalog No.:BCC6317
CAS No.:1404437-62-2
- Fmoc-Dap(Dnp)-OH
Catalog No.:BCC2666
CAS No.:140430-54-2
- 7-Methoxycoumarin-4-acetyl-P-L-G-L-β-(2,4-dinitrophenylamino)A-R amide
Catalog No.:BCC1086
CAS No.:140430-53-1
- Glycyrrhizic acid
Catalog No.:BCN5941
CAS No.:1405-86-3
- Bacitracin
Catalog No.:BCC4632
CAS No.:1405-87-4
- Bacitracin Zinc
Catalog No.:BCC4633
CAS No.:1405-89-6
- 1,2-Methylenedioxy-3,10,11-trimethoxynoraporphine
Catalog No.:BCN1573
CAS No.:14050-90-9
- Methyl chanofruticosinate
Catalog No.:BCN6223
CAS No.:14050-92-1
- Cassipourine
Catalog No.:BCN2154
CAS No.:14051-10-6
- 4-Ethylsyringol
Catalog No.:BCN3541
CAS No.:14059-92-8
- 12-Hydroxyjasmonic acid
Catalog No.:BCN6224
CAS No.:140631-27-2
- Kadsurenin D
Catalog No.:BCN6603
CAS No.:140669-89-2
- Levatin
Catalog No.:BCN2531
CAS No.:140670-84-4
- 1-Deoxydihydroceramide-1-sulfonic acid
Catalog No.:BCC4964
CAS No.:1407-03-0
- 4-Aza-5androstan-1-ene- 3-one-17carboxylic acid
Catalog No.:BCC8693
CAS No.:140700-63-6
Effects of dietary sunflower seeds and tylosin phosphate on production variables, carcass characteristics, fatty acid composition, and liver abscess incidence in crossbred steers.[Pubmed:18567724]
J Anim Sci. 2008 Nov;86(11):3125-36.
A 2 x 2 factorial experiment with 48 crossbred steers (with Hereford, Angus, and Charolais genetics, and an initial BW of 373 +/- 8.4 kg) was conducted to evaluate the effects of dietary sunflower seeds (SS) and Tylosin phosphate (TP) on production factors, carcass characteristics, liver abscess incidence, and fatty acid composition of the muscle (pars costalis diaphragmatis; PCD) and subcutaneous fat. Individually penned steers were fed either a control diet of 84.5% rolled barley, 14% barley silage, and 1.5% mineral and vitamin mix on a DM basis, or an SS diet, in which SS replaced 15% of the diet. Half the animals fed each diet received TP at 11 mg/kg of DM as a top dressing. Interactions were significant for all production factors. A reduction (P = 0.008) in DMI was observed from 10.1 +/- 0.4 kg/d, in steers fed the control diet, to 8.9 +/- 0.3 and 8.6 +/- 0.3 kg/d, in steers fed the SS and SS + TP diets, respectively. Greater (P = 0.014) ADG was observed for steers fed the control diet than for those fed the SS or SS + TP diet (1.4 vs. 1.1 and 1.2, SE = 0.1 kg/d, respectively); however, G:F ratios were greater (P = 0.011) in steers fed the control diets than in those fed the SS diets. Steers fed the control and SS diets had the heaviest and lightest HCW (347 +/- 6.9 vs. 325 +/- 8.4 kg; P = 0.025), respectively. Lean meat yield (%) of steers fed SS was greater (P = 0.117) than in steers fed the control diets, whereas total lean yield [(HCW x lean meat yield)/100] was similar (P = 0.755). Provision of the SS or SS + TP diet eliminated (P = 0.08 for interaction) liver abscesses compared with the 36 and 9% incidence in steers fed the control or control + TP diet, respectively. Fatty acid weight percentages (wt%) followed similar patterns in PCD and subcutaneous fat. Feeding the SS diets led to greater (P = 0.001) wt% of 18:0 and 18:2n-6, but reduced the wt% of 16:0, 9-cis (c)-18:1, and 18:3n-3 in PCD compared with that in steers fed the control diets, but the wt% of 9c,11-trans (t), and 10t,12c CLA were increased (P = 0.001) by 36 and 400% in PCD. Dietary SS increased (P < 0.001) the wt% of trans-18:1 isomers. The 10t-18:1 and 11t-18:1 isomers were the greatest, but dietary TP elevated (P = 0.004) only 10t-18:1, and total trans-18:1 (excluding 11t-18:1) was 0.47 +/- 0.06 g/100 g of PCD. Dietary SS for finishing steers reduced the incidence of liver abscesses without affecting total lean yield of the carcass, with modest increases in trans fatty acids and in potentially beneficial fatty acids (11t-18:1 and CLA).
Impact of tylosin phosphate and distillers dried grains with solubles on energy and nutrient digestibility and flow through the gastrointestinal tract in growing pigs.[Pubmed:24126274]
J Anim Sci. 2013 Dec;91(12):5687-95.
The objective of this study was to evaluate the impact of Tylosin phosphate (TP) on energy and nutrient digestibility and flow through the gastrointestinal tract in growing pigs fed corn-soybean meal or corn-soybean meal-distillers dried grains with solubles (DDGS) based diets. Eighteen barrows (initial BW = 32.6 +/- 1.2 kg) were surgically fitted with a T-cannula in the distal ileum and allotted to a Youden square design with 6 diets and 3 replicate periods. Treatments were arranged in a 2 x 2 factorial: TP (0 vs. 44 mg/kg) and DDGS (0 vs. 25%). Two N-free dietary treatments (0 vs. 44 mg/kg TP) were also included for determining basal ileal endogenous AA losses (IAAend) and the effect of TP on basal IAAend. Replicate periods included 4 d of adaptation to treatments and 2 sampling periods. Fecal collection occurred on d 5 and 6 and ileal digesta collection occurred on d 7 and 8 for sampling period 1 whereas sampling period 2 included fecal collection on d 11 and 12 and ileal digesta collection on d 13 and 14. Apparent ileal digestibility (AID) and apparent total tract digestibility (ATTD) were calculated for DM, energy, and NDF. The AID and standardized ileal digestibility (SID) of AA were calculated. Inclusion of DDGS reduced AID (68.0 vs. 72.8%; P < 0.001) and ATTD (79.9 vs. 85.0%; P < 0.001) of energy. There were no effects of TP on energy digestibility. The DDGS inclusion increased the amount of GE (1.47 vs. 1.18 Mcal/kg DMI; P < 0.001) and NDF (94 vs. 60 g/kg DMI; P < 0.001) remaining at the terminal ileum; however, hindgut disappearance of energy (0.55 vs. 0.53 Mcal/kg DMI) and NDF (13 vs. 15 g/kg DMI) was similar between the corn-soybean meal-DDGS and corn-soybean meal based diets. There were no effects of TP on basal IAAend; therefore, SID AA values were calculated using means of the 2 N-free diets. The SID of Lys (79.6 vs. 84.1%; P < 0.001) and all other indispensible AA, except Leu, was lower in the DDGS diets. Inclusion of TP did not influence SID of AA. In conclusion, under the conditions of this experiment, TP did not affect digestibility of AA or the digestibility and gastrointestinal tract flow of energy and the inclusion of DDGS did not affect the response to TP.
Effect of in-feed administration and withdrawal of tylosin phosphate on antibiotic resistance in enterococci isolated from feedlot steers.[Pubmed:26074889]
Front Microbiol. 2015 May 27;6:483.
Tylosin phosphate is a macrolide commonly administered to cattle in North America for the control of liver abscesses. This study investigated the effect of in-feed administration of Tylosin phosphate to cattle at subtherapeutic levels and its subsequent withdrawal on macrolide resistance using enterococci as an indicator bacterium. Fecal samples were collected from steers that received no antibiotics and steers administered Tylosin phosphate (11 ppm) in-feed for 197 days and withdrawn 28 days before slaughter. Enterococcus species isolated from fecal samples were identified through sequencing the groES-EL intergenic spacer region and subject to antimicrobial susceptibility testing, identification of resistance determinants and pulsed-field gel electrophoresis profiling. Tylosin increased (P < 0.05) the proportion of ery(R) and tyl(R) enterococci within the population. Just prior to its removal, the proportion of ery(R) and tyl(R) resistant enterococci began decreasing and continued to decrease after tylosin was withdrawn from the diet until there was no difference (P > 0.05) between treatments on d 225. This suggests that antibiotic withdrawal prior to slaughter contributes to a reduction in the proportion of macrolide resistant enterococci entering the food chain. Among the 504 enterococci isolates characterized, Enterococcus hirae was found to predominate (n = 431), followed by Enterococcus villorum (n = 32), Enterococcus faecium (n = 21), Enterococcus durans (n = 7), Enterococcus casseliflavus (n = 4), Enterococcus mundtii (n = 4), Enterococcus gallinarum (n = 3), Enterococcus faecalis (n = 1), and Enterococcus thailandicus (n = 1). The diversity of enterococci was greater in steers at arrival than at exit from the feedlot. Erythromycin resistant isolates harbored the erm(B) and/or msrC gene. Similar PFGE profiles of ery(R) E. hirae pre- and post-antibiotic treatment suggest that increased abundance of ery(R) enterococci after administration of Tylosin phosphate reflects selection for strains that were already present within the gastrointestinal tract of cattle at arrival.
Comparative pharmacokinetics and bioavailability of tylosin tartrate and tylosin phosphate after a single oral and i.v. administration in chickens.[Pubmed:24325541]
J Vet Pharmacol Ther. 2014 Jun;37(3):312-5.
The pharmacokinetics and oral bioavailability of tylosin tartrate and Tylosin phosphate were carried out in broiler chickens according to a principle of single dose, random, parallel design. The two formulations of tylosin were given orally and intravenously at a dose level of 10 mg/kg b.w to chicken after an overnight fasting (n = 10 chickens/group). Serial blood samples were collected at different time points up to 24 h postdrug administration. A high performance liquid chromatography method was used for the determination of tylosin concentrations in chicken plasma. The tylosin plasma concentration's time plot of each chicken was analyzed by the 3P97 software. The pharmacokinetics of tylosin was best described by a one-compartmental open model 1st absorption after oral administration. After intravenous administration the pharmacokinetics of tylosin was best described by a two-compartmental open model, and there were no significant differences between tylosin tartrate and Tylosin phosphate. After oral administration, there were significant differences in the Cmax (0.18 +/- 0.01, 0.44 +/- 0.09) and AUC (0.82 +/- 0.05, 1.57 +/- 0.25)between Tylosin phosphate and tylosin tartrate. The calculated oral bioavailability (F) of tylosin tartrate and Tylosin phosphate were 25.78% and 13.73%, respectively. Above all, we can reasonably conclude that, the absorption of tylosin tartrate is better than Tylosin phosphate after oral administration.


