UBP 282CAS# 544697-47-4 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
Number of papers citing our products

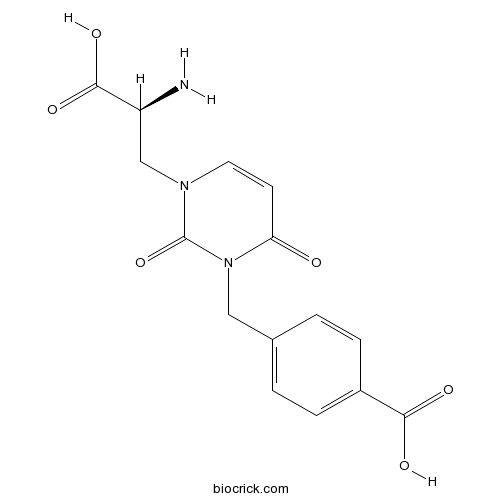
Chemical structure

3D structure
| Cas No. | 544697-47-4 | SDF | Download SDF |
| PubChem ID | 6604912 | Appearance | Powder |
| Formula | C15H15N3O6 | M.Wt | 333.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 3-CBW | ||
| Solubility | Soluble to 100 mM in 1eq. NaOH and to 25 mM in 1eq. HCl | ||
| Chemical Name | 4-[[3-[(2S)-2-amino-2-carboxyethyl]-2,6-dioxopyrimidin-1-yl]methyl]benzoic acid | ||
| SMILES | C1=CC(=CC=C1CN2C(=O)C=CN(C2=O)CC(C(=O)O)N)C(=O)O | ||
| Standard InChIKey | XLRLZPOBHPIDFX-NSHDSACASA-N | ||
| Standard InChI | InChI=1S/C15H15N3O6/c16-11(14(22)23)8-17-6-5-12(19)18(15(17)24)7-9-1-3-10(4-2-9)13(20)21/h1-6,11H,7-8,16H2,(H,20,21)(H,22,23)/t11-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AMPA and kainate receptor antagonist. Inhibits AMPA receptor-, but not kainate receptor-mediated currents on spinal neonatal motoneurons yet antagonizes kainate-induced responses on dorsal root C-fibres. |

UBP 282 Dilution Calculator

UBP 282 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0003 mL | 15.0015 mL | 30.003 mL | 60.006 mL | 75.0075 mL |
| 5 mM | 0.6001 mL | 3.0003 mL | 6.0006 mL | 12.0012 mL | 15.0015 mL |
| 10 mM | 0.3 mL | 1.5002 mL | 3.0003 mL | 6.0006 mL | 7.5008 mL |
| 50 mM | 0.06 mL | 0.3 mL | 0.6001 mL | 1.2001 mL | 1.5002 mL |
| 100 mM | 0.03 mL | 0.15 mL | 0.3 mL | 0.6001 mL | 0.7501 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MRS 1845
Catalog No.:BCC7198
CAS No.:544478-19-5
- c-di-AMP
Catalog No.:BCC8054
CAS No.:54447-84-6
- HIV-1 integrase inhibitor
Catalog No.:BCC1618
CAS No.:544467-07-4
- Capadenoson
Catalog No.:BCC1450
CAS No.:544417-40-5
- Norcantharidin
Catalog No.:BCN1281
CAS No.:5442-12-6
- Myristic acid
Catalog No.:BCN8390
CAS No.:544-63-8
- Palmitoylethanolamide
Catalog No.:BCC6828
CAS No.:544-31-0
- Lirinidine
Catalog No.:BCN8274
CAS No.:54383-28-7
- N,N-Bis(2-hydroxyethyl)-p-phenylenediamine sulphate
Catalog No.:BCN8366
CAS No.:54381-16-7
- 7-Hydroxy-5,8-dimethoxyflavanone
Catalog No.:BCN5722
CAS No.:54377-24-1
- BMS-538203
Catalog No.:BCC4136
CAS No.:543730-41-2
- 4'-Methoxyacetoacetanilide
Catalog No.:BCC8712
CAS No.:5437-98-9
- JNJ 10181457 dihydrochloride
Catalog No.:BCC7842
CAS No.:544707-20-2
- 3alpha-dihydrocadambine
Catalog No.:BCN8151
CAS No.:54483-84-0
- Jolkinolide E
Catalog No.:BCN3772
CAS No.:54494-34-7
- 5-Glutinen-3-ol
Catalog No.:BCN5723
CAS No.:545-24-4
- Uvaol
Catalog No.:BCN5724
CAS No.:545-46-0
- Lupeol
Catalog No.:BCN5725
CAS No.:545-47-1
- Erythrodiol
Catalog No.:BCN5726
CAS No.:545-48-2
- 5-Aminolevulinic acid HCl
Catalog No.:BCC4883
CAS No.:5451-09-2
- H-Leu-CMK.HCl
Catalog No.:BCC2971
CAS No.:54518-92-2
- Methyl protodioscin
Catalog No.:BCN6342
CAS No.:54522-52-0
- Methyl protogracillin
Catalog No.:BCN8177
CAS No.:54522-53-1
- Nicardipine HCl
Catalog No.:BCC4685
CAS No.:54527-84-3
Structural requirements for novel willardiine derivatives acting as AMPA and kainate receptor antagonists.[Pubmed:12684265]
Br J Pharmacol. 2003 Mar;138(6):1093-100.
1. The natural product willardiine is an AMPA receptor agonist. We have examined the structural changes required to convert willardiine into an antagonist at AMPA and kainate receptors. Structure-activity analysis has been carried out to discover the structural features required to increase the potency and/or selectivity of the antagonists at AMPA or kainate receptors. 2. Reduction of the fast component of the dorsal root-evoked ventral root potential (fDR-VRP) has been used to investigate AMPA receptor antagonist activity. To examine antagonist activity at kainate receptors, the ability of compounds to depress kainate-induced depolarisations of dorsal root fibres was assessed. 3. Blocking ionisation of the uracil ring by adding a methyl group to the N(3) position was not sufficient to convert willardiine into an antagonist. However, willardiine derivatives with a side-chain bearing a carboxylic acid group at the N(3)-position of the uracil ring could antagonise AMPA and kainate receptors. 4. S stereochemistry was optimal for antagonism. When compounds with differing interacidic group chain lengths were compared, a group chain length of two methylene groups was preferable for AMPA receptor antagonism in the series of compounds bearing a carboxyalkyl side chain (UBP275, UBP277 and UBP279 reduced the fDR-VRP with IC(50) values of 287+/-41, 23.8+/-3.9 and 136+/-17 micro M, respectively). For kainate receptor antagonism, two or three methylene groups were almost equally acceptable (UBP277 and UBP279 reduced dorsal root kainate responses with apparent K(D) values of 73.1+/-4.5 and 60.5+/-4.1 micro M, respectively). 5. Adding an iodo group to the 5-position of UBP277 and UBP282 enhanced activity at kainate receptors (UBP291 and UBP301 antagonised kainate responses on the dorsal root with apparent K(D) values of 9.83+/-1.62 and 5.94+/-0.63 micro M, respectively). 6. The most useful antagonist identified in this study was UBP301, which was a potent and approximately 30-fold selective kainate receptor antagonist. UBP282 may also be of use in isolating a non-GluR5-mediated kainate response.
The novel antagonist 3-CBW discriminates between kainate receptors expressed on neonatal rat motoneurones and those on dorsal root C-fibres.[Pubmed:12429586]
Br J Pharmacol. 2002 Dec;137(7):1125-33.
1. The natural product willardiine is a selective AMPA receptor agonist. We report that an N(3)-substituted analogue of willardiine, (S)-3-(4-carboxybenzyl)willardiine 3-CBW, antagonizes AMPA and kainate receptors expressed on motoneurones and dorsal root C-fibres, respectively. 2. Reduction of the fast component of the dorsal root-evoked ventral root potential (fDR-VRP) has been used as a novel method to compare AMPA receptor antagonists. 3-CBW, NBQX and GYKI53655 depressed the fDR-VRP with IC(50) values of 10.3+/-2.4, 0.214+/-0.043 and 4.03+/-0.31 micro M, respectively. That 3-CBW depressed the fDR-VRP by acting at AMPA and not metabotropic glutamate receptors was demonstrated by the lack of effect of LY341495 (100 micro M). 3. The Schild plot for antagonism of responses to (S)-5-fluorowillardiine on motoneurones by 3-CBW had a slope of 1.11+/-0.13 giving a pA(2) value of 4.48. The Schild plot for antagonism of kainate responses on the dorsal root by 3-CBW had a slope of 1.05+/-0.05 giving a pA(2) value of 4.96. 4. On neonatal rat motoneurones 3-CBW (200 micro M) almost completely abolished responses to AMPA while responses to NMDA, kainate and DHPG were 101.6+/-11.6%, 39.4+/-5.8% and 110.5+/-9.0% of control, respectively. 3-CBW can therefore be used to isolate kainate receptor responses from those mediated by AMPA receptors. 5 3-CBW antagonized kainate-induced responses on dorsal root C-fibres with a pA(2) value of 4.96 whereas kainate receptor mediated responses (isolated by including GYKI53655 in the medium) on motoneurones were not completely blocked by 200 micro M 3-CBW, substantiating evidence that kainate receptors on neonatal rat motoneurones differ from those on dorsal root C-fibres.


