XL765PI3K/mTOR inhibitor CAS# 1123889-87-1 |
- IPI-145 (INK1197)
Catalog No.:BCC1104
CAS No.:1201438-56-3
- IC-87114
Catalog No.:BCC1161
CAS No.:371242-69-2
- PI-103
Catalog No.:BCC1162
CAS No.:371935-74-9
- PIK-75
Catalog No.:BCC1163
CAS No.:372196-77-5
- TGX-221
Catalog No.:BCC1244
CAS No.:663619-89-4
Quality Control & MSDS
Number of papers citing our products

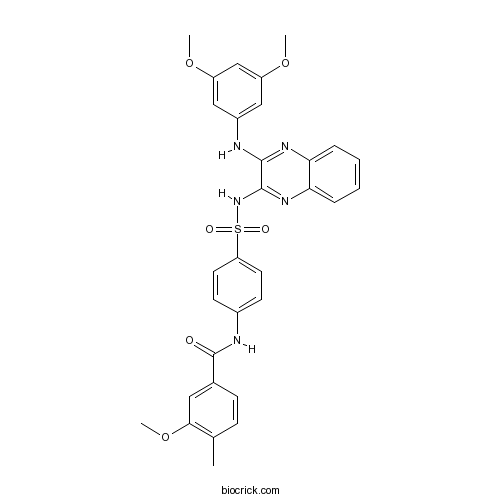
Chemical structure
3D structure
| Cas No. | 1123889-87-1 | SDF | Download SDF |
| PubChem ID | 49867926 | Appearance | Powder |
| Formula | C31H29N5O6S | M.Wt | 599.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SAR245409 (XL765);1349796-36-6 | ||
| Solubility | Soluble in DMSO | ||
| Chemical Name | N-[4-[[3-(3,5-dimethoxyanilino)quinoxalin-2-yl]sulfamoyl]phenyl]-3-methoxy-4-methylbenzamide | ||
| SMILES | CC1=C(C=C(C=C1)C(=O)NC2=CC=C(C=C2)S(=O)(=O)NC3=NC4=CC=CC=C4N=C3NC5=CC(=CC(=C5)OC)OC)OC | ||
| Standard InChIKey | HJSSPYJVWLTYHG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C31H29N5O6S/c1-19-9-10-20(15-28(19)42-4)31(37)33-21-11-13-25(14-12-21)43(38,39)36-30-29(34-26-7-5-6-8-27(26)35-30)32-22-16-23(40-2)18-24(17-22)41-3/h5-18H,1-4H3,(H,32,34)(H,33,37)(H,35,36) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | XL765 is an inhibitor of PI3K with IC50 values of 39, 113, 9, 43 and 157 nM for p110α, β, γ, δ and mTOR, respectively. | ||||||
| Targets | p110α | p110β | p110γ | p110δ | mTOR | ||
| IC50 | 39 nM | 113 nM | 9 nM | 43 nM | 157 nM | ||

XL765 Dilution Calculator

XL765 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6676 mL | 8.3379 mL | 16.6758 mL | 33.3517 mL | 41.6896 mL |
| 5 mM | 0.3335 mL | 1.6676 mL | 3.3352 mL | 6.6703 mL | 8.3379 mL |
| 10 mM | 0.1668 mL | 0.8338 mL | 1.6676 mL | 3.3352 mL | 4.169 mL |
| 50 mM | 0.0334 mL | 0.1668 mL | 0.3335 mL | 0.667 mL | 0.8338 mL |
| 100 mM | 0.0167 mL | 0.0834 mL | 0.1668 mL | 0.3335 mL | 0.4169 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
XL765 is a potent inhibitor of PI3Ks with IC50 values 39, 113,43,9 nM for p110α, β, δ, γ and 190, 908 nM for mTORC1, and mTORC2 respectively. [1]
The phosphoinositide-3 kinase (PI3K) pathway has been identified as an important target in breast cancer research. The PI3K pathway is integral to diverse cellular activities, including cellular metabolism and proliferation, survival, and differentiation. Besides, mTOR also acts as the nexus of signaling pathways regulating cell growth and proliferation. By inhibiting these targets, XL765 is thought to contribute to resistance to a variety of anticancer therapies. [2]
XL765 inhibits all four class I PI3K isoforms and mTOR with IC50 values in the nanomolar range in biochemical assays, whereas is highly selective against a panel of over 130 other human kinases. XL765 exhibits inhibition of PI3K-dependent production of the second messenger PIP3, and mTOR-dependent signaling stimulated by nutrient in cellular assays. Moreover, XL765 inhibits PI3K and mTOR-dependent phosphorylation of key components in the PI3K pathway including AKT, the substrates of AKT PRAS40 and GSK3β, p70S6K, the p70S6K substrate S6, and 4E-BP1 in diverse cancer cells. [1,3]
Treatment of XL765 to mice bearing xenografts of PIK3CA mutant MCF-7 breast adenocarcinoma cells or PTEN-deficient PC-3 prostate adenocarcinoma cells resulted in significant inhibition of PI3K and mTOR signaling. XL765 significantly decreased tumor growth or caused tumor shrinkage in different xenograft tumor models, including brain, lung, breast, ovarian, and prostate cancers which were correlated with inhibition of tumor cell proliferation and tumor angiogenesis, and with induction of apoptosis. However, rapamycin inhibited proliferation caused little or no induction of apoptosis. These results demonstrate that a dual inhibitor strategy, targeting both PI3K and mTOR, may offer significant advantages over specifically targeting the mTOR/Raptor complex. XL765 is currently undergoing a Phase I clinical trial in patients with solid tumors.[3]
References:
1.Braña I, LoRusso P, Baselga J, et al. A Phase 1 dose-escalation study of the safety, pharmacokinetics and pharmacodynamics of XL765 (SAR245409), a PI3K/TORC1/TORC2 inhibitor administered orally to patients with advanced malignancies[J]. bid, 2010, 16: 5.
2.Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer[J]. The oncologist, 2011, 16(Supplement 1): 12-19.
3.Laird A D. XL765 targets tumor growth, survival, and angiogenesis in preclinical models by dual inhibition of PI3K and mTOR[J]. Molecular Cancer Therapeutics, 2007, 6(11 Supplement): B250-B250.
- DMPQ dihydrochloride
Catalog No.:BCC6977
CAS No.:1123491-15-5
- WAY-262611
Catalog No.:BCC5507
CAS No.:1123231-07-1
- BRL 52537 hydrochloride
Catalog No.:BCC6751
CAS No.:112282-24-3
- H-Asp(OcHex)-OH
Catalog No.:BCC2887
CAS No.:112259-66-2
- 20(R)-Ginsenoside Rh2
Catalog No.:BCN2484
CAS No.:112246-15-8
- (S)-tert-Leucinol
Catalog No.:BCN8367
CAS No.:112245-13-3
- Stigmastane-3,6-diol
Catalog No.:BCN6003
CAS No.:112244-29-8
- 16-O-Methyl-14,15-didehydroisovincanol
Catalog No.:BCN1618
CAS No.:112237-71-5
- 14,15-Didehydrovincamenine
Catalog No.:BCN6002
CAS No.:112219-48-4
- Pam3CSK4
Catalog No.:BCC6245
CAS No.:112208-00-1
- DMAP
Catalog No.:BCC2842
CAS No.:1122-58-3
- Ikshusterol 3-O-glucoside
Catalog No.:BCN6001
CAS No.:112137-81-2
- Tetramethylpyrazine
Catalog No.:BCN1008
CAS No.:1124-11-4
- 3-Deoxysappanchalcone
Catalog No.:BCN3736
CAS No.:112408-67-0
- 3'-Deoxy-4-O-methylsappanol
Catalog No.:BCN3675
CAS No.:112408-68-1
- 2',5,7-Trihydroxy-8-methoxyflavanone
Catalog No.:BCN6004
CAS No.:112408-71-6
- Ganoderic acid TN
Catalog No.:BCN2443
CAS No.:112430-64-5
- Ganoderic acid T-Q
Catalog No.:BCN3209
CAS No.:112430-66-7
- Ganoderic acid Jb
Catalog No.:BCN7972
CAS No.:112430-68-9
- AZ3146
Catalog No.:BCC3731
CAS No.:1124329-14-1
- 3-Phenyl-1-(pyrrol-1-yl)propan-1-one
Catalog No.:BCN4005
CAS No.:112448-69-8
- U-54494A hydrochloride
Catalog No.:BCC6668
CAS No.:112465-94-8
- H-Glu(OcHex)-OH
Catalog No.:BCC2929
CAS No.:112471-82-6
- 4,4-Pentamethylenepiperidine hydrochloride
Catalog No.:BCC6059
CAS No.:1125-01-5
Dual PI3K/mTOR inhibitor, XL765 (SAR245409), shows superior effects to sole PI3K [XL147 (SAR245408)] or mTOR [rapamycin] inhibition in prostate cancer cell models.[Pubmed:26219891]
Tumour Biol. 2016 Jan;37(1):341-51.
Deregulation of phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathway contributes to prostate cancer development and progression. Here, we compared the in vitro effects of the dual PI3K/mTOR inhibitor (XL765) with those observed with the sole PI3K (XL147) or mTOR (rapamycin) inhibition in 2 non-tumor prostate epithelial cell lines, 8 prostate cancer cell lines, and 11 prostate cancer cell derivatives. We demonstrated that the XL765 treatment showed superior and proliferative effects of XL147 or rapamycin. The XL765 effects were associated to increasing the chromosome region maintenance 1 (CRM1)-mediated nuclear localization of glycogen synthase kinase 3 beta (GSK3beta) and Foxo-1a with higher induction of apoptosis when compared to those observed in XL147 and rapamycin treatments. IC50 values were calculated in phosphatase and tensin homologue deleted on chromosome 10 (PTEN)-positive and PTEN-negative cell lines as well as after PTEN transfection or PTEN downmodulation by siRNA strategy revealing that the presence of this protein was associated with reduced sensitivity to PI3K and mTOR inhibitors. The comparison of IC50 values was also calculated for androgen-dependent and -independent cell lines as well as after androgen receptor (AR) transfection or the AR downmodulation by siRNA strategy revealing that androgen independence was associated with enhanced responsiveness. Our results provide a rationale to use the dual PI3K/Akt/mTOR inhibitors in hormone-insensitive prostate cancer models due to the overactivity of PI3K/Akt/mTOR in this disease condition.
The pan phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor SAR245409 (voxtalisib/XL765) blocks survival, adhesion and proliferation of primary chronic lymphocytic leukemia cells.[Pubmed:26338274]
Leukemia. 2016 Feb;30(2):337-45.
The phosphoinositide 3-kinases (PI3Ks) are critical components of the B-cell receptor (BCR) pathway and have an important role in the pathobiology of chronic lymphocytic leukemia (CLL). Inhibitors of PI3Kdelta block BCR-mediated cross-talk between CLL cells and the lymph node microenvironment and provide significant clinical benefit to CLL patients. However, the PI3Kdelta inhibitors applied thus far have limited direct impact on leukemia cell survival and thus are unlikely to eradicate the disease. The use of inhibitors of multiple isoforms of PI3K might lead to deeper remissions. Here we demonstrate that the pan-PI3K/mammalian target of rapamycin inhibitor SAR245409 (voxtalisib/XL765) was more pro-apoptotic to CLL cells--irrespective of their ATM/p53 status--than PI3Kalpha or PI3Kdelta isoform selective inhibitors. Furthermore, SAR245409 blocked CLL survival, adhesion and proliferation. Moreover, SAR245409 was a more potent inhibitor of T-cell-mediated production of cytokines, which support CLL survival. Taken together, our in vitro data provide a rationale for the evaluation of a pan-PI3K inhibitor in CLL patients.
Phase I dose-escalation study of the PI3K/mTOR inhibitor voxtalisib (SAR245409, XL765) plus temozolomide with or without radiotherapy in patients with high-grade glioma.[Pubmed:26019185]
Neuro Oncol. 2015 Sep;17(9):1275-83.
BACKGROUND: This phase I study aimed to evaluate safety, maximum tolerated dose, pharmacokinetics, pharmacodynamics, and preliminary efficacy of voxtalisib (SAR245409, XL765), a pan-class I phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) inhibitor, in combination with temozolomide (TMZ), with or without radiation therapy (RT), in patients with high-grade glioma. METHODS: Patients received voxtalisib 30-90 mg once daily (q.d.) or 20-50 mg twice daily (b.i.d.), in combination with 200 mg/m(2) TMZ (n = 49), or voxtalisib 20 mg q.d. with 75 mg/m(2) TMZ and RT (n = 5). A standard 3 + 3 dose-escalation design was used to determine the maximum tolerated dose. Patients were evaluated for adverse events (AEs), plasma pharmacokinetics, pharmacodynamic effects in skin biopsies, and tumor response. RESULTS: The maximum tolerated doses were 90 mg q.d. and 40 mg b.i.d. for voxtalisib in combination with TMZ. The most frequently reported treatment-related AEs were nausea (48%), fatigue (43%), thrombocytopenia (26%), and diarrhea (24%). The most frequently reported treatment-related grade >/=3 AEs were lymphopenia (13%), thrombocytopenia, and decreased platelet count (9% each). Pharmacokinetic parameters were similar to previous studies with voxtalisib monotherapy. Moderate inhibition of PI3K signaling was observed in skin biopsies. Best response was partial response in 4% of evaluable patients, with stable disease observed in 68%. CONCLUSIONS: Voxtalisib in combination with TMZ with or without RT in patients with high-grade gliomas demonstrated a favorable safety profile and a moderate level of PI3K/mTOR pathway inhibition.


