ZIPPKMζ inhibitor CAS# 863987-12-6 |
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Chelerythrine chloride
Catalog No.:BCN8322
CAS No.:3895-92-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 863987-12-6 | SDF | Download SDF |
| PubChem ID | 16156119 | Appearance | Powder |
| Formula | C90H154N30O17 | M.Wt | 1928.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | z-Pseudosubstrate inhibitory peptide | ||
| Solubility | Soluble to 1 mg/ml in water | ||
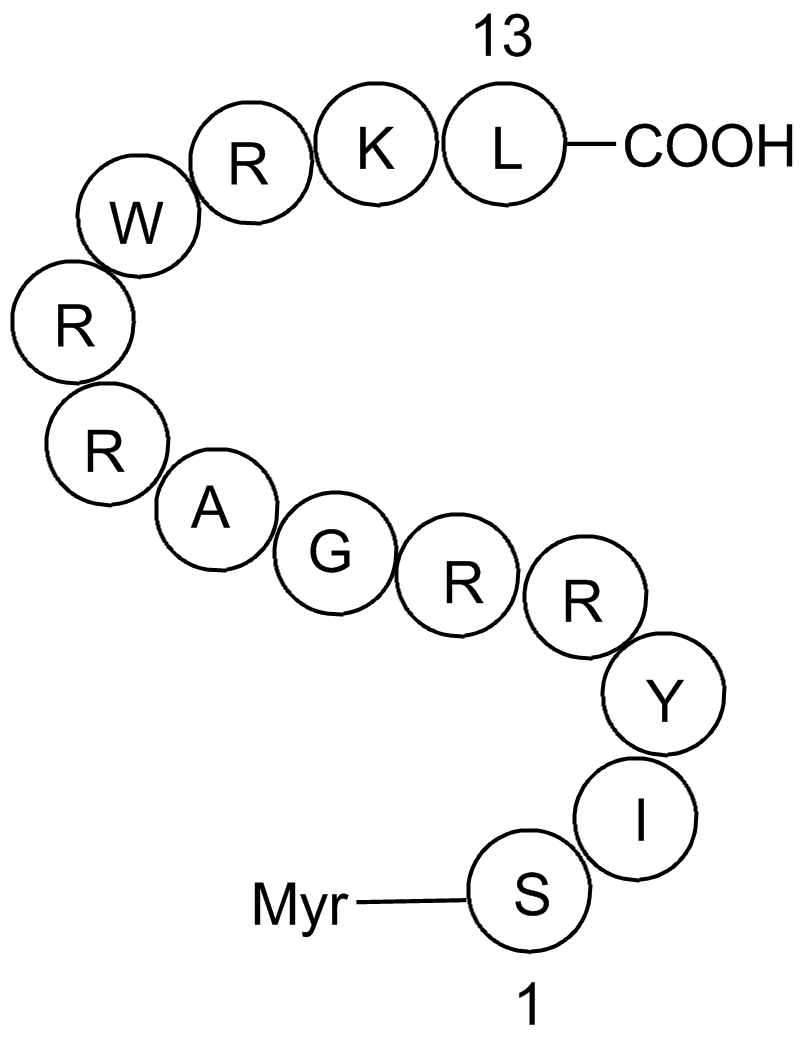
| Sequence | SIYRRGARRWRKL (Modifications: Ser-1 = Myr-Ser) | ||
| SMILES | CCCCCCCCCCCCCC(=O)NC(CO)C(=O)NC(C(C)CC)C(=O)NC(CC1=CC=C(C=C1)O)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCCNC(=N)N)C(=O)NCC(=O)NC(C)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCCNC(=N)N)C(=O)NC(CC2=CNC3=CC=CC=C32)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCCCN)C(=O)NC(CC(C)C)C(=O)O | ||
| Standard InChIKey | CRKARHQCXWSUMV-HOHDCHNJSA-N | ||
| Standard InChI | InChI=1S/C90H154N30O17/c1-7-9-10-11-12-13-14-15-16-17-18-36-71(123)110-70(52-121)83(134)120-73(54(5)8-2)84(135)118-67(48-56-37-39-58(122)40-38-56)81(132)115-64(33-25-44-104-88(96)97)77(128)112-61(31-23-42-102-86(92)93)75(126)108-51-72(124)109-55(6)74(125)111-63(32-24-43-103-87(94)95)76(127)114-66(35-27-46-106-90(100)101)79(130)117-68(49-57-50-107-60-29-20-19-28-59(57)60)82(133)116-65(34-26-45-105-89(98)99)78(129)113-62(30-21-22-41-91)80(131)119-69(85(136)137)47-53(3)4/h19-20,28-29,37-40,50,53-55,61-70,73,107,121-122H,7-18,21-27,30-36,41-49,51-52,91H2,1-6H3,(H,108,126)(H,109,124)(H,110,123)(H,111,125)(H,112,128)(H,113,129)(H,114,127)(H,115,132)(H,116,133)(H,117,130)(H,118,135)(H,119,131)(H,120,134)(H,136,137)(H4,92,93,102)(H4,94,95,103)(H4,96,97,104)(H4,98,99,105)(H4,100,101,106)/t54-,55-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,73-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Novel, cell-permeable inhibitor of protein kinase Mζ (PKMζ), a constitutively active, atypical PKC isozyme involved in LTP maintenance. Selectively blocks PKMζ-induced synaptic potentiation in hippocampal slices in vitro. Reverses late-phase LTP (IC50 = 1 - 2.5 μM) and produces persistent loss of 1-day-old spatial memory following central administration in vivo. Control and biotinylated peptides also available. |

ZIP Dilution Calculator

ZIP Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: By suppressing protein kinase Mξ (PKMξ), ZIP reverses late-phase LTP with an IC50 of 1 - 2.5 μM.
Long-term potentiation (LTP), a persistent synaptic enhancement, is considered to be a substrate for memory. A typical LTP process includes two phases, induction and maintenance. PKMξ as an active form of protein kinase C (PKC) isozyme is necessary and potent for LTP maintenance. ZIP is applied as a novel and cell-permeable inhibitor for PKMξ and can therefore block LTP. [1]
In vitro: In order to determine the specific phase of LTP affected by PKMξ, ZIP was added at different concentrations to the bath before cells incubation. Experiments with ZIP were compared with experiments without the peptide. This study showed that ZIP could selectively inhibit PKMξ-induced synaptic potentiation in hippocampal slices in vitro. [2]
In vivo: ZIP was the first tool available to test the maintenance hypothesis in vitro. The effect of ZIP on late phase of LTP in vivo was also detected using the rat hippocampus. It was found that intra-hippocampal injection of ZIP with a dosage of 10 nmol in 1 ml saline rapidly reversed the late-phase LTP and led to persistent loss of 1-day-old spatial memory. [3]
Clinical trial: So far, no clinical trial has been conducted.
References:
[1]Ling SF, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF and Sacktor TC. Protein kinase Mξ is necessary and sufficient for LTP maintenance. Nat. Neurosci. 2002 Apr. 5(4): 2956.
[2]Serrano P, Yao Y and Sacktor TC. Persistent phosphorylation by protein kinase maintains late-phase long-term potentiation. J. Neurosci. 2005 Feb. 25(8): 1979–84.
[3]Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006 Aug. 313: 1141-4.
- Mc-MMAE
Catalog No.:BCC5201
CAS No.:863971-24-8
- Methoxy-X04
Catalog No.:BCC6331
CAS No.:863918-78-9
- Fluconazole
Catalog No.:BCC4905
CAS No.:86386-73-4
- Methyl diacetoxy-6-gingerdiol
Catalog No.:BCN3268
CAS No.:863780-90-9
- Diacetoxy-4-gingerdiol
Catalog No.:BCN3337
CAS No.:863780-88-5
- Ganoderic acid X
Catalog No.:BCN7971
CAS No.:86377-53-9
- Ganoderic acid Y
Catalog No.:BCN2439
CAS No.:86377-52-8
- 5,8-Epidioxyergosta-6,9(11),22-trien-3-ol
Catalog No.:BCN1327
CAS No.:86363-50-0
- 6-Epiharpagide
Catalog No.:BCN4563
CAS No.:86362-16-5
- Gnetol
Catalog No.:BCN3382
CAS No.:86361-55-9
- Cebranopadol
Catalog No.:BCC1467
CAS No.:863513-91-1
- Medetomidine HCl
Catalog No.:BCC4351
CAS No.:86347-15-1
- Empagliflozin (BI 10773)
Catalog No.:BCC2472
CAS No.:864070-44-0
- GSK429286A
Catalog No.:BCC2532
CAS No.:864082-47-3
- AMG 548
Catalog No.:BCC6084
CAS No.:864249-60-5
- C 021 dihydrochloride
Catalog No.:BCC6047
CAS No.:864289-85-0
- BNTX maleate
Catalog No.:BCC6838
CAS No.:864461-31-4
- Sanggenone K
Catalog No.:BCN3373
CAS No.:86450-77-3
- Sanggenone H
Catalog No.:BCN2946
CAS No.:86450-80-8
- Nigrolineaxanthone V
Catalog No.:BCN4411
CAS No.:864516-31-4
- Sequosempervirin B
Catalog No.:BCN4777
CAS No.:864719-17-5
- Sequosempervirin D
Catalog No.:BCN4562
CAS No.:864719-19-7
- SB 699551
Catalog No.:BCC7594
CAS No.:864741-95-7
- TC-MCH 7c
Catalog No.:BCC6149
CAS No.:864756-35-4
Postmyelotomy Closure of Spinal Cord-"Zip Lock Technique": An Initial Experience.[Pubmed:28087435]
World Neurosurg. 2017 Apr;100:261-266.
Proper closure of the pia matter is necessary to restore normal anatomy and prevent postoperative painful dysesthesia after excision of intramedullary spinal cord tumor. Two methods of closure of the pia have been described: welding technique and conventional suturing. Here, we report our initial experience with a new "pial press" or "ZIP lock" technique for pial closure, where pial layers are simply held together and plunged into each other with small microtooth forceps. Advantages of the technique over other techniques are it has less chance of suture-related complications or trauma to the posterior column and the simplicity of the technique.
Drosophila dorsal closure: An orchestra of forces to zip shut the embryo.[Pubmed:28077304]
Mech Dev. 2017 Apr;144(Pt A):2-10.
Dorsal closure, a late-embryogenesis process, consists in the sealing of an epidermal gap on the dorsal side of the Drosophila embryo. Because of its similarities with wound healing and neural tube closure in humans, it has been extensively studied in the last twenty years. The process requires the coordination of several force generating mechanisms, that together will ZIP shut the epidermis. Recent works have provided a precise description of the cellular behavior at the origin of these forces and proposed quantitative models of the process. In this review, we will describe the different forces acting in dorsal closure. We will present our current knowledge on the mechanisms generating and regulating these forces and report on the different quantitative mathematical models proposed so far.
Genome-wide characterization and expression profiling of HD-Zip gene family related to abiotic stress in cassava.[Pubmed:28249019]
PLoS One. 2017 Mar 1;12(3):e0173043.
Homeodomain-leucine ZIPper (HD-ZIP) gene family plays important roles in various abiotic stresses and hormone signaling in plants. However, no information is currently available regarding this family in cassava (Manihot esculenta), an important drought-tolerant crop in tropical and sub-tropical areas. Here, 57 HD-ZIP genes (MeHDZ01-57) were identified in the cassava genome, and they were classified into four subfamilies based on phylogenetic analysis, which was further supported by their gene structure and conserved motif characteristics. Of which five gene pairs were involved in segmental duplication but none for tandem duplication, suggesting that segmental duplication was the main cause for the expansion of MeHDZ gene family in cassava. Global expression profiles revealed that MeHDZ genes were constitutively expressed, or not expressed, or tissue-specific expressed in examined tissues in both cultivated and wild subspecies. Transcriptomic analysis of three genotypes showed that most of MeHDZ genes responded differently to drought and polyethylene glycol treatments. Subsequently, quantitative RT-PCR analysis revealed comprehensive responses of twelve selected MeHDZ genes to various stimuli including cold, salt, and ABA treatments. These findings will increase our understanding of HD-ZIP gene family involved in abiotic stresses and signaling transduction, and will provide a solid base for further functional characterization of MeHDZ genes in cassava.
Genome-wide analysis of the HD-ZIP IV transcription factor family in Gossypium arboreum and GaHDG11 involved in osmotic tolerance in transgenic Arabidopsis.[Pubmed:28251315]
Mol Genet Genomics. 2017 Jun;292(3):593-609.
HD-ZIP IV proteins belong to the homeodomain-leucine ZIPper (HD-ZIP) transcription factor family and are involved in trichome development and drought stress in plants. Although some functions of the HD-ZIP IV group are well understood in Arabidopsis, little is known about their function in cotton. In this study, HD-ZIP genes were identified from three Gossypium species (G. arboreum, G. raimondii and G. hirsutum) and clustered into four families (HD-ZIP I, II, III and IV) to separate HD-ZIP IV from the other three families. Systematic analyses of phylogeny, gene structure, conserved domains, and expression profiles in different plant tissues and the expression patterns under osmotic stress in leaves were further conducted in G. arboreum. More importantly, ectopic overexpression of GaHDG11, a representative of the HD-ZIP IV family, confers enhanced osmotic tolerance in transgenic Arabidopsis plants, possibly due to elongated primary root length, lower water loss rates, high osmoprotectant proline levels, significant levels of antioxidants CAT, and/or SOD enzyme activity with reduced levels of MDA. Taken together, these observations may lay the foundation for future functional analysis of cotton HD-ZIP IV genes to unravel their biological roles in cotton.
Storage of spatial information by the maintenance mechanism of LTP.[Pubmed:16931766]
Science. 2006 Aug 25;313(5790):1141-4.
Analogous to learning and memory storage, long-term potentiation (LTP) is divided into induction and maintenance phases. Testing the hypothesis that the mechanism of LTP maintenance stores information requires reversing this mechanism in vivo and finding out whether long-term stored information is lost. This was not previously possible. Recently however, persistent phosphorylation by the atypical protein kinase C isoform, protein kinase Mzeta (PKMz), has been found to maintain late LTP in hippocampal slices. Here we show that a cell-permeable PKMz inhibitor, injected in the rat hippocampus, both reverses LTP maintenance in vivo and produces persistent loss of 1-day-old spatial information. Thus, the mechanism maintaining LTP sustains spatial memory.
Persistent phosphorylation by protein kinase Mzeta maintains late-phase long-term potentiation.[Pubmed:15728837]
J Neurosci. 2005 Feb 23;25(8):1979-84.
Protein kinase Mzeta (PKMzeta), an autonomously active atypical PKC isoform, is both necessary and sufficient for enhanced synaptic transmission during long-term potentiation (LTP) maintenance. LTP, however, evolves through several temporal phases, which may be mediated by distinct molecular mechanisms of potentiation. Here, we determined the specific phase of LTP maintained by PKMzeta. Using a selective, cell-permeable zeta-pseudosubstrate inhibitor at concentrations that block potentiation produced by postsynaptic perfusion of PKMzeta, we inhibited PKMzeta activity at various times after tetanization of Schaffer collateral/commissural-CA1 synapses. Inhibition of PKMzeta did not affect baseline AMPA receptor-mediated synaptic transmission or an early phase of LTP. In contrast, the inhibitor reversed established LTP when applied 1, 3, or 5 h after tetanic stimulation. Control nontetanized pathways within the hippocampal slices were unaffected. An inactive scrambled version of the peptide had no effect on LTP. Thus, persistent, increased phosphorylation by PKMzeta specifically maintains the late phase of LTP.


