c-JUN peptidePeptide inhibitor of JNK/c-Jun interaction CAS# 610273-01-3 |
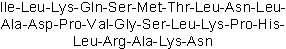
- Ki20227
Catalog No.:BCC1678
CAS No.:623142-96-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure
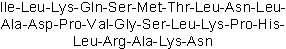
3D structure
| Cas No. | 610273-01-3 | SDF | Download SDF |
| PubChem ID | 90488732 | Appearance | Powder |
| Formula | C121H210N36O34S | M.Wt | 2745.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
| Sequence | ILKQSMTLNLADPVGSLKPHLRAKN | ||
| SMILES | CCC(C)C(C(=O)NC(CC(C)C)C(=O)NC(CCCCN)C(=O)NC(CCC(=O)N)C(=O)NC(CO)C(=O)NC(CCSC)C(=O)NC(C(C)O)C(=O)NC(CC(C)C)C(=O)NC(CC(=O)N)C(=O)NC(CC(C)C)C(=O)NC(C)C(=O)NC(CC(=O)O)C(=O)N1CCCC1C(=O)NC(C(C)C)C(=O)NCC(=O)NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CCCCN)C(=O)N2CCCC2C(=O)NC(CC3=CNC=N3)C(=O)NC(CC(C)C)C(=O)NC(CCCNC(=N)N)C(=O)NC(C)C(=O)NC(CCCCN)C(=O)NC(CC(=O)N)C(=O)O)N | ||
| Standard InChIKey | OVPPMIXGVMXSNS-CLIBVNCNSA-N | ||
| Standard InChI | InChI=1S/C121H210N36O34S/c1-19-65(14)94(128)115(185)149-79(48-62(8)9)106(176)139-71(30-21-24-39-123)100(170)141-73(35-36-89(125)161)102(172)153-86(57-159)112(182)142-74(37-44-192-18)103(173)155-96(68(17)160)117(187)150-80(49-63(10)11)108(178)147-82(51-90(126)162)110(180)144-76(45-59(2)3)104(174)136-67(16)98(168)151-83(53-93(165)166)119(189)157-43-28-34-88(157)114(184)154-95(64(12)13)116(186)133-55-92(164)137-85(56-158)111(181)146-78(47-61(6)7)107(177)143-75(31-22-25-40-124)118(188)156-42-27-33-87(156)113(183)148-81(50-69-54-131-58-134-69)109(179)145-77(46-60(4)5)105(175)140-72(32-26-41-132-121(129)130)99(169)135-66(15)97(167)138-70(29-20-23-38-122)101(171)152-84(120(190)191)52-91(127)163/h54,58-68,70-88,94-96,158-160H,19-53,55-57,122-124,128H2,1-18H3,(H2,125,161)(H2,126,162)(H2,127,163)(H,131,134)(H,133,186)(H,135,169)(H,136,174)(H,137,164)(H,138,167)(H,139,176)(H,140,175)(H,141,170)(H,142,182)(H,143,177)(H,144,180)(H,145,179)(H,146,181)(H,147,178)(H,148,183)(H,149,185)(H,150,187)(H,151,168)(H,152,171)(H,153,172)(H,154,184)(H,155,173)(H,165,166)(H,190,191)(H4,129,130,132)/t65-,66-,67-,68+,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,86-,87-,88-,94-,95-,96-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Peptide comprising residues 33 - 57 of the JNK binding (δ) domain of human c-Jun. Disrupts JNK/c-Jun interaction leading to inhibition of serum-induced c-Jun phosphorylation, up-regulation of p21cip/waf and modulation of inflammatory gene expression. Specifically induces apoptosis in HeLa tumor cells. |

c-JUN peptide Dilution Calculator

c-JUN peptide Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ferulamide
Catalog No.:BCN4129
CAS No.:61012-31-5
- Succinylcholine Chloride Dihydrate
Catalog No.:BCC4564
CAS No.:6101-15-1
- Isoacetovanillone
Catalog No.:BCN7166
CAS No.:6100-74-9
- Tenuazonic acid
Catalog No.:BCN1859
CAS No.:610-88-8
- H-Leu-OH
Catalog No.:BCC2968
CAS No.:61-90-5
- Zoxazolamine
Catalog No.:BCC4751
CAS No.:61-80-3
- 4-Aminohippuric Acid
Catalog No.:BCC4753
CAS No.:61-78-9
- Phenylephrine HCl
Catalog No.:BCC4335
CAS No.:61-76-7
- Mefenamic Acid
Catalog No.:BCC4433
CAS No.:61-68-7
- Papaverine Hydrochloride
Catalog No.:BCC8348
CAS No.:61-25-6
- Adenosine 5'-monophosphate
Catalog No.:BCC8809
CAS No.:61-19-8
- Dibucaine (Cinchocaine) HCl
Catalog No.:BCC3760
CAS No.:61-12-1
- Teicoplanin
Catalog No.:BCC4731
CAS No.:61036-62-2
- N-Desmethylclozapine
Catalog No.:BCC6887
CAS No.:6104-71-8
- L-Thyroxine sodium salt pentahydrate
Catalog No.:BCC4283
CAS No.:6106-07-6
- Boc-His(Nτ-Me)-OH
Catalog No.:BCC2684
CAS No.:61070-22-2
- Isobonducellin
Catalog No.:BCN4130
CAS No.:610778-85-3
- Icotinib
Catalog No.:BCC4473
CAS No.:610798-31-7
- 4-Phenyl-1,2,3,4-tetrahydroisoquinoline hydrochloride
Catalog No.:BCC6769
CAS No.:6109-35-9
- Tectoridin
Catalog No.:BCN1020
CAS No.:611-40-5
- (R)-Mandelic acid
Catalog No.:BCN8532
CAS No.:611-71-2
- Bromhexine hydrochloride
Catalog No.:BCC8898
CAS No.:611-75-6
- Epipterosin L 2'-O-glucoside
Catalog No.:BCN4614
CAS No.:61117-89-3
- 3,9-Dihydroxypterocarpan
Catalog No.:BCN4131
CAS No.:61135-91-9
Peptide-DNA conjugates as tailored bivalent binders of the oncoprotein c-Jun.[Pubmed:25778494]
Org Biomol Chem. 2015 May 21;13(19):5385-90.
We describe a ds-oligonucleotide-peptide conjugate that is able to efficiently dismount preformed DNA complexes of the bZIP regions of oncoproteins c-Fos and c-Jun (AP-1), and therefore might be useful as disrupters of AP-1-mediated gene expression pathways.
Anti-tumor peptide AP25 decreases cyclin D1 expression and inhibits MGC-803 proliferation via phospho-extracellular signal-regulated kinase-, Src-, c-Jun N-terminal kinase- and phosphoinositide 3-kinase-associated pathways.[Pubmed:26063313]
Mol Med Rep. 2015 Sep;12(3):4396-4402.
The anti-tumor peptide AP25 is a prototype integrin antagonist, which exhibits antiangiogenic and antitumor activity. The molecular mechanisms by which AP25 inhibits the growth of the MGC803 gastric carcinoma cell line were investigated in the present study. Kras specific RNA interference by lentiviral infection was successfully induced in MGC803 cells [MGC803 short hairpin (sh)RNA group] and the expression levels of Kras, phosphorylated extracellular signalregulated kinase (p-ERK) and cyclin D1 were observed to be markedly decreased. By contrast, AP25 caused cell cycle arrest of intact MGC803 cells and decreased pERK and cyclin D1 expression levels. Of note, 0.43.2 microM AP25 no longer inhibited MGC803 shRNA growth, indicating that AP25, at such concentrations, exerts its effect mainly through the Ras/Raf/mitogen-activated protein kinase kinase/ERK pathway, whereas at 25 microM, AP25 was able to inhibit MGC803 shRNA growth. Chemical inhibitors of Src, cJun Nterminal kinase (JNK) and phosphoinositide 3kinase (PI3K) were used to confirm that 25 microM AP25 inhibited growth of cells in the MGC803 shRNA group and activated intracellular signaling pathways with Src, JNK and PI3K as key enzymes. In conclusion, the present study revealed the signal transduction pathways activated by AP25 at low (0.43.2 microM) or high (25 microM) concentrations. It also confirmed that integrins, when interacting with the freely moving ligand AP25 instead of immobilized extracellular matrix glycoproteins, are able to initiate cell signaling via similar pathways as in the latter case but with a reversed effect, to inhibit cell growth.
ZAK induces cardiomyocyte hypertrophy and brain natriuretic peptide expression via p38/JNK signaling and GATA4/c-Jun transcriptional factor activation.[Pubmed:25869677]
Mol Cell Biochem. 2015 Jul;405(1-2):1-9.
Cardiomyocyte hypertrophy is an adaptive response of heart to various stress conditions. During the period of stress accumulation, transition from physiological hypertrophy to pathological hypertrophy results in the promotion of heart failure. Our previous studies found that ZAK, a sterile alpha motif and leucine zipper containing kinase, was highly expressed in infarcted human hearts and demonstrated that overexpression of ZAK induced cardiac hypertrophy. This study evaluates, cellular events associated with the expression of two doxycycline (Dox) inducible Tet-on ZAK expression systems, a Tet-on ZAK WT (wild-type), and a Tet-on ZAK DN (mutant, Dominant-negative form) in H9c2 myoblast cells; Tet-on ZAK WT was found to increase cell size and hypertrophic marker BNP in a dose-dependent manner. To ascertain the mechanism of ZAK-mediated hypertrophy, expression analysis with various inhibitors of the related upstream and downstream proteins was performed. Tet-on ZAK WT expression triggered the p38 and JNK pathway and also activated the expression and nuclear translocation of p-GATA4 and p-c-Jun transcription factors, without the involvement of p-ERK or NFATc3. However, Tet-on ZAK DN showed no effect on the p38 and JNK signaling cascade. The results showed that the inhibitors of JNK1/2 and p38 significantly suppressed ZAK-induced BNP expression. The results show the role of ZAK and/or the ZAK downstream events such as JNK and p38 phosphorylation, c-Jun, and GATA-4 nuclear translocation in cardiac hypertrophy. ZAK and/or the ZAK downstream p38, and JNK pathway could therefore be potential targets to ameliorate cardiac hypertrophy symptoms in ZAK-overexpressed patients.
Scorpion Venom Heat-Resistant Peptide Attenuates Glial Fibrillary Acidic Protein Expression via c-Jun/AP-1.[Pubmed:26134308]
Cell Mol Neurobiol. 2015 Nov;35(8):1073-9.
Scorpion venom has been used in the Orient to treat central nervous system diseases for many years, and the protein/peptide toxins in Buthus martensii Karsch (BmK) venom are believed to be the effective components. Scorpion venom heat-resistant peptide (SVHRP) is an active component of the scorpion venom extracted from BmK. In a previous study, we found that SVHRP could inhibit the formation of a glial scar, which is characterized by enhanced glial fibrillary acidic protein (GFAP) expression, in the epileptic hippocampus. However, the cellular and molecular mechanisms underlying this process remain to be clarified. The results of the present study indicate that endogenous GFAP expression in primary rat astrocytes was attenuated by SVHRP. We further demonstrate that the suppression of GFAP was primarily mediated by inhibiting both c-Jun expression and its binding with AP-1 DNA binding site and other factors at the GFAP promoter. These results support that SVHRP contributes to reducing GFAP at least in part by decreasing the activity of the transcription factor AP-1. In conclusion, the effects of SVHRP on astrocytes with respect to the c-Jun/AP-1 signaling pathway in vitro provide a practical basis for studying astrocyte activation and inhibition and a scientific basis for further studies of traditional medicine.
Disruption of the c-JUN-JNK complex by a cell-permeable peptide containing the c-JUN delta domain induces apoptosis and affects a distinct set of interleukin-1-induced inflammatory genes.[Pubmed:12832416]
J Biol Chem. 2003 Oct 10;278(41):40213-23.
The transcription factor activator protein (AP)-1 plays crucial roles in proliferation, cell death, and the immune response. c-JUN is an important component of AP-1, but only very few c-JUN response genes have been identified to date. Activity of c-JUN is controlled by NH2-terminal phosphorylation (JNP) of its transactivation domain by a family of JUN-NH2-terminal protein kinases (JNK). JNK form a stable complex with c-JUN in vitro and in vivo. We have targeted this interaction by means of a cell-permeable peptide containing the JNK-binding (delta) domain of human c-JUN. This peptide strongly and specifically induced apoptosis in HeLa tumor cells, which was paralleled by inhibition of serum-induced c-JUN phosphorylation and up-regulation of the cell cycle inhibitor p21cip/waf. Application of the c-JUN peptide to interleukin (IL)-1-stimulated human primary fibroblasts resulted in up-regulation of four genes, namely COX-2, MnSOD, I kappa B alpha, and MAIL and down-regulation of 10 genes, namely CCL8, mPGES, SAA1, hIAP-1, hIAP-2, pent(r)axin-3, CXCL10, IL-1 beta, ICAM-1, and CCL2. Only a small group of genes, namely pent(r)axin-3, CXCL10, ICAM-1, and IL-1 beta, was inhibited by both the c-JUN peptide and the JNK inhibitor SP600125. Thereby, and by additional experiments using small interfering RNA to suppress endogenous c-JUN we identify for the first time three distinct groups of inflammatory genes whose IL-1-induced expression depends on c-JUN, on JNK, or on both. These results shed further light on the complexity of c-JUN-JNK-mediated gene regulation and also highlight the potential use of dissecting signaling downstream from JNK to specifically target proliferative diseases or the inflammatory response.


