(S)-(-)-HA-966CAS# 111821-58-0 |
- Deuterated Atazanivir-D3-3
Catalog No.:BCC2117
CAS No.:1092540-52-7
- Deuterated Atazanivir-D3-1
Catalog No.:BCC2115
CAS No.:1092540-56-1
- Amprenavir (agenerase)
Catalog No.:BCC3619
CAS No.:161814-49-9
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- BMS-538203
Catalog No.:BCC4136
CAS No.:543730-41-2
- BMS-707035
Catalog No.:BCC2133
CAS No.:729607-74-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 111821-58-0 | SDF | Download SDF |
| PubChem ID | 183351 | Appearance | Powder |
| Formula | C4H8N2O2 | M.Wt | 116.12 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
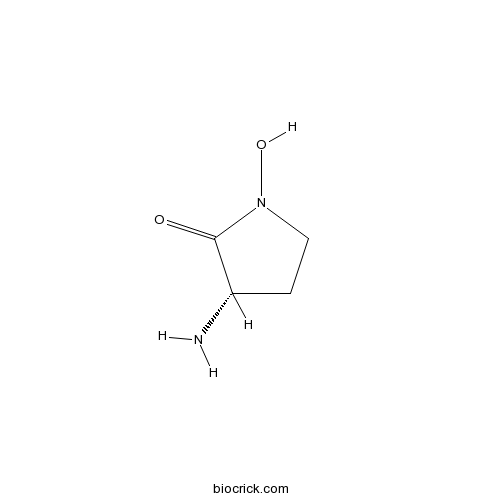
| Chemical Name | (3S)-3-amino-1-hydroxypyrrolidin-2-one | ||
| SMILES | C1CN(C(=O)C1N)O | ||
| Standard InChIKey | HCKUBNLZMKAEIN-VKHMYHEASA-N | ||
| Standard InChI | InChI=1S/C4H8N2O2/c5-3-1-2-6(8)4(3)7/h3,8H,1-2,5H2/t3-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Possesses potent sedative and ataxic action, probably through disruption of striatal dopaminergic mechanisms. R-enantiomer also available. |

(S)-(-)-HA-966 Dilution Calculator

(S)-(-)-HA-966 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.6118 mL | 43.0589 mL | 86.1178 mL | 172.2356 mL | 215.2945 mL |
| 5 mM | 1.7224 mL | 8.6118 mL | 17.2236 mL | 34.4471 mL | 43.0589 mL |
| 10 mM | 0.8612 mL | 4.3059 mL | 8.6118 mL | 17.2236 mL | 21.5295 mL |
| 50 mM | 0.1722 mL | 0.8612 mL | 1.7224 mL | 3.4447 mL | 4.3059 mL |
| 100 mM | 0.0861 mL | 0.4306 mL | 0.8612 mL | 1.7224 mL | 2.1529 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-Glu-Oet.HCl
Catalog No.:BCC2685
CAS No.:1118-89-4
- Demethoxyfumitremorgin C
Catalog No.:BCN7240
CAS No.:111768-16-2
- Remacemide hydrochloride
Catalog No.:BCC7129
CAS No.:111686-79-4
- Elastase Inhibitor
Catalog No.:BCC1225
CAS No.:111682-13-4
- GSK1838705A
Catalog No.:BCC3787
CAS No.:1116235-97-2
- Cyanidin-3-O-arabinoside chloride
Catalog No.:BCN3023
CAS No.:111613-04-8
- Anonamine
Catalog No.:BCN2139
CAS No.:111566-66-6
- 3',5-Dihydroxy-4',5',6,7-tetramethoxyflavone
Catalog No.:BCN1620
CAS No.:111537-41-8
- Fmoc-D-Phg-OH
Catalog No.:BCC3316
CAS No.:111524-95-9
- Nyasicol
Catalog No.:BCN5999
CAS No.:111518-95-7
- Nyasicoside
Catalog No.:BCN5998
CAS No.:111518-94-6
- Metformin HCl
Catalog No.:BCC4799
CAS No.:1115-70-4
- 10-O-Methylprotosappanin B
Catalog No.:BCN6599
CAS No.:111830-77-4
- Hancinone C
Catalog No.:BCN4751
CAS No.:111843-10-8
- UCPH 101
Catalog No.:BCC7692
CAS No.:1118460-77-7
- BIM 23042
Catalog No.:BCC5998
CAS No.:111857-96-6
- 2,4-Dihydroxyphenylacetyl-L-asparagine
Catalog No.:BCC6585
CAS No.:111872-98-1
- KY 02111
Catalog No.:BCC3628
CAS No.:1118807-13-8
- H-Glu(OEt)-OH
Catalog No.:BCC2930
CAS No.:1119-33-1
- H-Arg-OH.HCl
Catalog No.:BCC2857
CAS No.:1119-34-2
- 2-Guanidinoethanesulfinic acid
Catalog No.:BCN1800
CAS No.:1119-54-6
- MitMAB
Catalog No.:BCC7892
CAS No.:1119-97-7
- (1S,3R)-ACPD
Catalog No.:BCC6590
CAS No.:111900-32-4
- Temocapril
Catalog No.:BCC5013
CAS No.:111902-57-9
Pertussis toxin lesions of the rat substantia nigra block the inhibitory effects of the gamma-hydroxybutyrate agent, S(-)HA-966 without affecting the basal firing properties of dopamine neurons.[Pubmed:10516961]
Neuropsychopharmacology. 1999 Nov;21(5):650-61.
S(-)3-amino-1-hydroxypyrrolidone-2 (S(-)HA-966), a potent gamma-hydroxybutyrate-like drug, inhibits spontaneous firing and induces a pacemaker-like discharge pattern in nigral dopamine (DA)-containing neurons. Recent evidence has suggested that these effects could be mediated by GABAB receptors and, thus, is likely to involve G protein intermediaries. To test this hypothesis, extracellular single-unit recording techniques were used to assess the effects of S(-)HA-966 in animals that had received an intranigral injection of pertussis toxin (PT). Failure to respond to the inhibitory effects of apomorphine was taken as presumptive evidence that PT-sensitive G protein-coupled receptors had been inactivated. No significant differences were observed in the basal firing properties of DA cells recorded in control and PT-lesioned animals. However, in marked contrast to the inhibitory effects observed in uninjected and sham-lesioned animals, S(-)HA-966 significantly increased the firing rate of apomorphine-insensitive DA neurons in PT-lesioned rats. The excitatory effects of S(-)HA-966 were accompanied by a significant reduction in bursting activity and an increase in the regularity of firing. These data indicate that the inhibitory effects of S(-)HA-966 are mediated locally within the substantia nigra by a PT-sensitive substrate, presumably a G protein-coupled receptor.
(S)-(-)-HA-966, a gamma-hydroxybutyrate-like agent, prevents enhanced mesocorticolimbic dopamine metabolism and behavioral correlates of restraint stress, conditioned fear and cocaine sensitization.[Pubmed:9353390]
J Pharmacol Exp Ther. 1997 Nov;283(2):712-21.
This report investigates the effect of the negative enantiomer of 1-hydroxy-3-aminopyrrolidone-2 (HA-966) on behavioral and biochemical changes elicited by pharmacological or experimental paradigms which activate mesocorticolimbic dopaminergic neurotransmission. Several paradigms were used, including cocaine sensitization and two stressors: restraint for 30 min and an aversive conditioning model. (S)-(-)-HA-966 (3 and 5 mg/kg i.p.) prevented restraint stress-induced dopamine utilization in both the medial prefrontal cortex and nucleus accumbens, in contrast to the positive enantiomer. Conditioned fear increased dopamine metabolism in both the core and shell subdivisions of the nucleus accumbens, an effect blocked by (S)-(-)-HA-966. The conditioned stress-induced increase in dopamine metabolism in the medial prefrontal cortex was also blocked by (S)-(-)-HA-966. In addition, (S)-(-)-HA-966 suppressed fear-induced behaviors: immobility and defecation. In other studies, (S)-(-)-HA-966 (3 mg/kg i.p.) prevented locomotor sensitization without altering the acute motoric response elicited by cocaine. The highest dose of (S)-(-)-HA-966 (5 mg/kg i.p.) blocked acute cocaine-induced locomotion but resulted in sedation. In addition, the highest dose of (S)-(-)-HA-966 tested suppressed weight gain in control rats, unlike its enantiomer, (R)-(+)-HA-966. Because (S)-(-)-HA-966 has been proposed to act at the gamma-aminobutyric acid (GABA)B receptor, we examined the ability of (S)-(-) and (R)-(+)-HA-966 to displace [3H]-(-)-baclofen from cortical membranes to assess GABAB receptor binding. Neither enantiomer significantly altered [3H]-(-)-baclofen binding at relevant concentrations, indicating the actions of (S)-(-)-HA-966 reported here are the results of a mechanism apparently independent of the baclofen binding site on the GABAB receptor.
Responses of rat substantia nigra dopamine-containing neurones to (-)-HA-966 in vitro.[Pubmed:9051293]
Br J Pharmacol. 1997 Feb;120(4):575-80.
1. Extracellular single unit recording techniques were used to compare the effects of (-)-3-amino-1-hydroxypyrrolidin-2-one ((-)-HA-966) and (+/-)-baclofen on the activity of dopamine-containing neurones in 300 microns slices of rat substantia nigra. Electrophysiological data were compared with the outcome of in vitro binding experiments designed to assess the affinity of (-)-HA-966 for gamma-aminobutyric acid (GABAB) receptors. 2. Bath application of (-)-HA-966 produced a concentration-dependent inhibition of dopaminergic neuronal firing (EC50 = 444.0 microM; 95% confidence interval: 277.6 microM - 710.1 microM, n = 27) which was fully reversible upon washout from the recording chamber. Although similar effects were observed in response to (+/-)-baclofen, the direct-acting GABAB receptor agonist proved to be considerably more potent than (-)-HA-966 (EC50 = 0.54 microM; 95% confidence interval: 0.44 microM - 0.66 microM, n = 29) in vitro. 3. Low concentrations of chloral hydrate (10 microM) were without effect on the basal firing rate of nigral dopaminergic neurones but significantly increased the inhibitory effects produced by concomitant application of (-)-HA-966. 4. The inhibitory effects of (-)-HA-966 were completely reversed in the presence of the GABAB receptor antagonists, CGP-35348 (100 microM) and 2-hydroxysaclofen (500 microM). Bath application of CGP-35348 alone increased basal firing rate. However, the magnitude of the excitation (9.2 +/- 0.3%) was not sufficient to account for the ability of the antagonist to reverse fully the inhibitory effects of (-)-HA-966. 5. (-)-HA-966 (0.1-1.0 mM) produced a concentration-dependent displacement of [3H]-GABA from synaptic membranes in the presence of isoguvacine (40 microM). However, the affinity of the drug for GABAB binding sites was significantly less than that of GABA (0.0005 potency ratio) and showed no apparent stereoselectivity. 6. These results indicate that while (-)-HA-966 appears to act as a direct GABAB receptor agonist in vitro, its affinity for this receptor site is substantially less than that of GABA or baclofen and unlikely to account for the depressant actions of this drug which occur at levels approximately ten fold lower in vivo.
(+-)-1-hydroxy-3-aminopyrrolidone-2 (HA-966) inhibits the activity of substantia nigra dopamine neurons through a non-N-methyl-D-aspartate receptor-mediated mechanism.[Pubmed:1578355]
J Pharmacol Exp Ther. 1992 May;261(2):387-94.
(+-)-1-hydroxy-3-aminopyrrolidone-2 [(+/-)-HA-966] is known to cause a rapid and selective increase in striatal dopamine (DA) levels--an effect that has been attributed to the compound's presumed ability to block the spontaneous electrical activity of nigrostriatal DA neurons. In the present series of experiments, extracellular single unit recording techniques were used to explore this premise in the chloral hydrate-anesthetized rat and to compare the pharmacological effects of the racemic form of HA-966 with its resolved enantiomers. Systemic administration of (+/-)-HA-966 produced a dose-dependent inhibition in the firing rate of DA neurons in the zona compacta of the substantia nigra. The highest dose tested (40 mg/kg i.v.) completely inhibited the spontaneous activity of all cells tested. Pretreatment with naloxone (5 or 10 mg/kg i.v.) reduced the initial rate of decline in firing rate and the duration of inhibition but did not prevent a single dose of 40 mg/kg of (+/-)-HA-966 from totally inhibiting DA cell impulse flow. Systemic administration of the (-)-enantiomer of HA-966 (30 mg/kg i.v.) inhibited neuronal activity in a manner analogous to a single injection of 40 mg/kg of (+/-)-HA-966. Comparable doses of the (+)-enantiomer failed to affect significantly the firing rate of substantia nigra DA neurons but suppressed bursting activity and "normalized" neuronal discharge pattern.(ABSTRACT TRUNCATED AT 250 WORDS)
Enantiomers of HA-966 (3-amino-1-hydroxypyrrolid-2-one) exhibit distinct central nervous system effects: (+)-HA-966 is a selective glycine/N-methyl-D-aspartate receptor antagonist, but (-)-HA-966 is a potent gamma-butyrolactone-like sedative.[Pubmed:2153294]
Proc Natl Acad Sci U S A. 1990 Jan;87(1):347-51.
The antagonist effect of (+/-)-3-amino-1-hydroxypyrrolid-2-one (HA-966) at the N-methyl-D-aspartate (NMDA) receptor occurs through a selective interaction with the glycine modulatory site within the receptor complex. When the enantiomers of (+/-)-HA-966 were resolved, the (R)-(+)-enantiomer was found to be a selective glycine/NMDA receptor antagonist, a property that accounts for its anticonvulsant activity in vivo. In contrast, the (S)-(-)-enantiomer was only weakly active as an NMDA-receptor antagonist, but nevertheless it possessed a marked sedative and muscle relaxant action in vivo. In radioligand binding experiments, (+)-HA-966 inhibited strychnine-insensitive [3H]glycine binding to rat cerebral cortex synaptic membranes with an IC50 of 12.5 microM, whereas (-)-HA-966 had an IC50 value of 339 microM. In electrophysiological experiments, (+)-HA-966 selectively antagonized NMDA receptor responses in rat cortical slices, whereas the (-)-enantiomer was much weaker. On cultured cortical neurones (+)-HA-966 inhibited glycine-potentiated NMDA responses with an IC50 = 13 microM compared with (-)-HA-966, which has an IC50 = 708 microM. In agreement with findings with racemic HA-966, even high concentrations of (+)-HA-966 did not completely inhibit NMDA responses, suggesting that (+)-HA-966 is a low-efficacy partial agonist. (+)-HA-966 produced parallel shifts to the right of the glycine concentration curve for potentiation of NMDA responses, resulting in an estimated pKb = 5.6. In mice, (+)-HA-966 antagonized sound and N-methyl-DL-aspartic acid (NMDLA)-induced seizures with ED50 values of 52.6 mg/kg of body weight (i.p.) and 900 mg/kg (i.v.), respectively. The coadministration of D-serine dose-dependently (10-100 micrograms into the cerebral ventricles per mouse) antagonized the anticonvulsant effect of a submaximal dose of (+)-HA-966 (100 micrograms administered directly into the cerebral ventricles) against NMDLA-induced seizures. The sedative/ataxic effect of racemic HA-966 was mainly attributable to the (-)-enantiomer, which was greater than 25-fold more potent than the (+)-enantiomer. It is suggested that, as in the case of the sedative gamma-butyrolactone, disruption of striatal dopaminergic mechanisms may be responsible for this action.


