TemocaprilCAS# 111902-57-9 |
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Rosuvastatin Calcium
Catalog No.:BCC3853
CAS No.:147098-20-2
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 111902-57-9 | SDF | Download SDF |
| PubChem ID | 71323 | Appearance | Powder |
| Formula | C23H28N2O5S2 | M.Wt | 476.61 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
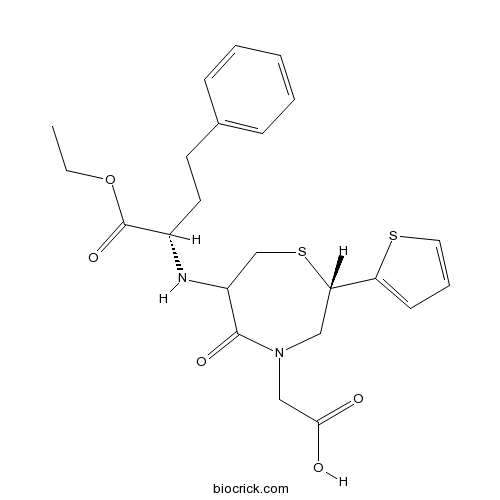
| Chemical Name | 2-[(2S)-6-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]-5-oxo-2-thiophen-2-yl-1,4-thiazepan-4-yl]acetic acid | ||
| SMILES | CCOC(=O)C(CCC1=CC=CC=C1)NC2CSC(CN(C2=O)CC(=O)O)C3=CC=CS3 | ||
| Standard InChIKey | FIQOFIRCTOWDOW-DXCJPMOASA-N | ||
| Standard InChI | InChI=1S/C23H28N2O5S2/c1-2-30-23(29)17(11-10-16-7-4-3-5-8-16)24-18-15-32-20(19-9-6-12-31-19)13-25(22(18)28)14-21(26)27/h3-9,12,17-18,20,24H,2,10-11,13-15H2,1H3,(H,26,27)/t17-,18?,20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Temocapril Dilution Calculator

Temocapril Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0982 mL | 10.4908 mL | 20.9815 mL | 41.963 mL | 52.4538 mL |
| 5 mM | 0.4196 mL | 2.0982 mL | 4.1963 mL | 8.3926 mL | 10.4908 mL |
| 10 mM | 0.2098 mL | 1.0491 mL | 2.0982 mL | 4.1963 mL | 5.2454 mL |
| 50 mM | 0.042 mL | 0.2098 mL | 0.4196 mL | 0.8393 mL | 1.0491 mL |
| 100 mM | 0.021 mL | 0.1049 mL | 0.2098 mL | 0.4196 mL | 0.5245 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Temocapril
- (1S,3R)-ACPD
Catalog No.:BCC6590
CAS No.:111900-32-4
- MitMAB
Catalog No.:BCC7892
CAS No.:1119-97-7
- 2-Guanidinoethanesulfinic acid
Catalog No.:BCN1800
CAS No.:1119-54-6
- H-Arg-OH.HCl
Catalog No.:BCC2857
CAS No.:1119-34-2
- H-Glu(OEt)-OH
Catalog No.:BCC2930
CAS No.:1119-33-1
- KY 02111
Catalog No.:BCC3628
CAS No.:1118807-13-8
- 2,4-Dihydroxyphenylacetyl-L-asparagine
Catalog No.:BCC6585
CAS No.:111872-98-1
- BIM 23042
Catalog No.:BCC5998
CAS No.:111857-96-6
- UCPH 101
Catalog No.:BCC7692
CAS No.:1118460-77-7
- Hancinone C
Catalog No.:BCN4751
CAS No.:111843-10-8
- 10-O-Methylprotosappanin B
Catalog No.:BCN6599
CAS No.:111830-77-4
- (S)-(-)-HA-966
Catalog No.:BCC6589
CAS No.:111821-58-0
- Adenanthin
Catalog No.:BCN6000
CAS No.:111917-59-0
- Quetiapine
Catalog No.:BCC1877
CAS No.:111974-69-7
- Quetiapine fumarate
Catalog No.:BCN5339
CAS No.:111974-72-2
- 2-Undecanone
Catalog No.:BCN8461
CAS No.:112-12-9
- Acetic acid octyl ester
Catalog No.:BCN8303
CAS No.:112-14-1
- Methyl hexadecanoate
Catalog No.:BCN8290
CAS No.:112-39-0
- Methyl Stearate
Catalog No.:BCN8309
CAS No.:112-61-8
- Methyl Oleate
Catalog No.:BCN8306
CAS No.:112-62-9
- Methyl linoleate
Catalog No.:BCN8137
CAS No.:112-63-0
- Oleic acid
Catalog No.:BCN7159
CAS No.:112-80-1
- Docosanoic acid
Catalog No.:BCC8952
CAS No.:112-85-6
- OctMAB
Catalog No.:BCC7893
CAS No.:1120-02-1
Promoter Polymorphism of RGS2 Gene Is Associated with Change of Blood Pressure in Subjects with Antihypertensive Treatment: The Azelnidipine and Temocapril in Hypertensive Patients with Type 2 Diabetes Study.[Pubmed:20981351]
Int J Hypertens. 2010 Aug 24;2010:196307.
We performed a prospective study to examine the genetic effect on the response to a calcium (Ca) channel blocker, azelnidipine and an ACE inhibitor, Temocapril treatment in patients with hypertension, as a part of the prior clinical trial, the Azelnidipine and Temocapril in Hypertensive Patients with Type 2 Diabetes Study (ATTEST). Methods and Results. All subjects who gave informed consent for genetic research were divided into two groups: the subjects treated with azelnidipine or Temocapril, for 52 weeks. We selected 18 susceptible genes for hypertension and determined their genotypes using TaqMan PCR method. RNA samples were extracted from peripheral blood, and quantitative real time PCR for all genes was performed using TaqMan method. One of the polymorphisms of the RGS2 gene was extracted as being able to influence the effect of these treatments to reduce BP. At eight weeks, BP change showed a significant interaction between the A-638G polymorphism of Regulator of G protein signaling-2 (RGS2) gene and treatment with azelnidipine or Temocapril. There was no gene whose expression was associated with BP phenotypes or the polymorphisms of each gene. Conclusions. A-638G polymorphism of the RGS-2 gene could be a predictive factor for therapeutic performance of Ca channel blockers.
Prediction of human intestinal absorption of the prodrug temocapril by in situ single-pass perfusion using rat intestine with modified hydrolase activity.[Pubmed:21474683]
Drug Metab Dispos. 2011 Jul;39(7):1263-9.
Intestinal absorption of Temocapril, a prodrug of Temocaprilat, was evaluated in an in situ rat jejunal perfusion model under various conditions of luminal pH and in the presence and absence of carboxylesterase-mediated hydrolysis. Temocapril was more easily taken up by mucosal cells at a luminal pH of 5.4 than at pH 6.4 or 7.4 and was extensively hydrolyzed to Temocaprilat in mucosal cells. The hydrolysis was limited by the intrinsic clearance and the influx rate at luminal perfusate pHs of 5.4 and 7.4, respectively. Temocaprilat, derived from Temocapril, was transported into both mesenteric vein and jejunal lumen according to pH partition theory. The net absorption of both Temocapril and Temocaprilat was highest at a luminal perfusate pH of 5.4. When both the luminal and venous fluid were at pH 7.4, Temocaprilat was transported approximately 3-fold faster into the lumen than into the vein, due presumably to the greater surface area of the brush border membrane because of the presence of microvilli. Under carboxylesterase-inhibited conditions, the hydrolysis of Temocapril was inhibited by only 50%. It is postulated that serine esterases located on the membranes of the epithelial cells were responsible for the residual hydrolysis. We have confirmed that Temocapril is most easily absorbed in the proximal intestine after meals, due to prolongation of the gastric emptying time, the lower intraluminal pH caused by secretion of bile acid, and the interaction between serine esterases and the digesta.
Matrix metalloproteinase-2 inhibition by temocapril and its important role in peritoneal transport.[Pubmed:23013132]
Clin Exp Pharmacol Physiol. 2012 Oct;39(10):864-8.
1. Matrix metalloproteinase (MMP)-2 plays an important role in tissue remodelling during peritoneal injury caused by peritoneal dialysis (PD), but MMP-2 inhibitors have not yet been used clinically. Recently, it was reported that captopril, an angiotensin-converting enzyme inhibitor (ACEI), can inhibit MMP-2. 2. To investigate the potential usefulness of ACEI during PD, the molecular interaction between the MMP-2 active site and the active form of Temocapril (Temocaprilat) was investigated using molecular modelling. Furthermore, the effects of Temocapril on MMP-2 activity in peritoneal effluents and the peritoneal solute transport rate of PD patients were determined. 3. Temocaprilat bound to the MMP-2 active centre and recognized two hydrophobic substrate-binding sites in the MMP-2 molecular model. Matrix metalloproteinase-2 activity in peritoneal effluents was directly inhibited by Temocaprilat (IC(50) 0.47 mumol/L). In one patient given Temocapril, the peritoneal solute transport rate decreased gradually during PD. 4. Temocapril may prove to be an important candidate for development as a novel therapeutic agent for MMP-2 inhibition to prevent peritoneal injury caused by PD.
Evaluation of transport mechanism of prodrugs and parent drugs formed by intracellular metabolism in Caco-2 cells with modified carboxylesterase activity: temocapril as a model case.[Pubmed:21618543]
J Pharm Sci. 2011 Sep;100(9):3985-94.
The intestinal absorption mechanism of Temocapril, an ester-type prodrug of Temocaprilat, was evaluated using Caco-2 cell monolayers with or without active carboxylesterase (CES)-mediated hydrolysis. The inhibition of CES-mediated hydrolysis was achieved by pretreatment of the monolayers with bis-p-nitrophenyl phosphate (BNPP), which inhibited 94% of the total hydrolysis of Temocapril in the Caco-2 cells. The remaining 6% hydrolysis was due to the presence of serine esterases, other than CES, on the cell membranes. Transport experiments under CES-inhibited conditions showed Temocapril not to be a substrate for peptide transporter 1 (PEPT1) or organic anion transporting polypeptides (OATPs), but to be an inhibitor of PEPT1; P-glycoprotein (P-gp) and breast-cancer-resistant protein (BCRP) were responsible for the efflux of Temocapril, which was mainly absorbed by passive diffusion at low apical pH. In Caco-2 cell monolayers with CES-mediated hydrolysis intact, Temocaprilat derived from Temocapril, was 2.5-fold more rapidly transported into the apical compartment than into the basolateral compartment due to the presence of microvilli on the apical membrane. In contrast, Temocaprilat at low intracellular concentrations, was preferentially transported across the basolateral membrane under CES-inhibited conditions.


