2,2-Bis(hydroxymethyl)butyric acidCAS# 10097-02-6 |
Quality Control & MSDS
Number of papers citing our products

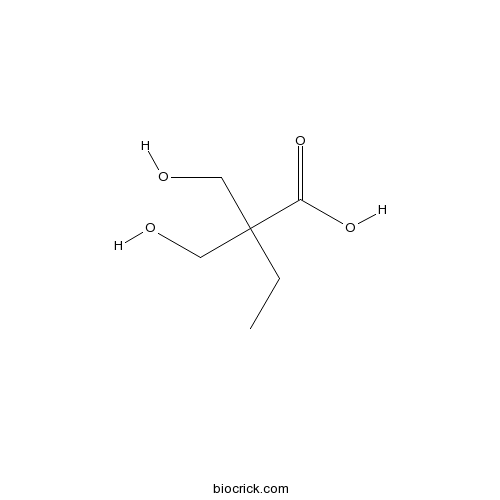
Chemical structure

3D structure
| Cas No. | 10097-02-6 | SDF | Download SDF |
| PubChem ID | 3301396 | Appearance | Powder |
| Formula | C6H12O4 | M.Wt | 148 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2,2-bis(hydroxymethyl)butanoic acid | ||
| SMILES | CCC(CO)(CO)C(=O)O | ||
| Standard InChIKey | JVYDLYGCSIHCMR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H12O4/c1-2-6(3-7,4-8)5(9)10/h7-8H,2-4H2,1H3,(H,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2,2-Bis(hydroxymethyl)butyric acid Dilution Calculator

2,2-Bis(hydroxymethyl)butyric acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.7568 mL | 33.7838 mL | 67.5676 mL | 135.1351 mL | 168.9189 mL |
| 5 mM | 1.3514 mL | 6.7568 mL | 13.5135 mL | 27.027 mL | 33.7838 mL |
| 10 mM | 0.6757 mL | 3.3784 mL | 6.7568 mL | 13.5135 mL | 16.8919 mL |
| 50 mM | 0.1351 mL | 0.6757 mL | 1.3514 mL | 2.7027 mL | 3.3784 mL |
| 100 mM | 0.0676 mL | 0.3378 mL | 0.6757 mL | 1.3514 mL | 1.6892 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Stachyose
Catalog No.:BCN2566
CAS No.:10094-58-3
- Caulophyllumine A
Catalog No.:BCN7928
CAS No.:1009318-60-8
- AZD2014
Catalog No.:BCC3732
CAS No.:1009298-59-2
- AZD8055
Catalog No.:BCC3629
CAS No.:1009298-09-2
- Panamycin 607
Catalog No.:BCN1813
CAS No.:100905-89-3
- Piceatannol
Catalog No.:BCN5824
CAS No.:10083-24-6
- (RS)-CPP
Catalog No.:BCC6561
CAS No.:100828-16-8
- 4,4'-Bis(α,α-dimethylbenzyl)diphenylamine
Catalog No.:BCC8661
CAS No.:10081-67-1
- Chlorahololide C
Catalog No.:BCN7256
CAS No.:1007859-25-7
- (R)-5-Hydroxy-1,7-diphenyl-3-heptanone
Catalog No.:BCN3591
CAS No.:100761-20-4
- Ganoderic acid M
Catalog No.:BCN2871
CAS No.:100761-17-9
- 1-O-Deacetylkhayanolide E
Catalog No.:BCN5823
CAS No.:1007387-95-2
- Rotundine
Catalog No.:BCN5983
CAS No.:10097-84-4
- Ebrotidine
Catalog No.:BCC1542
CAS No.:100981-43-9
- Thiamet G
Catalog No.:BCC4864
CAS No.:1009816-48-1
- NSC 687852 (b-AP15)
Catalog No.:BCC2389
CAS No.:1009817-63-3
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- Levofloxacin
Catalog No.:BCC4791
CAS No.:100986-85-4
- CY 208-243
Catalog No.:BCC6991
CAS No.:100999-26-6
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- Hyoscyamine
Catalog No.:BCN1946
CAS No.:101-31-5
- Bis[4-(dimethylamino)phenyl]methane
Catalog No.:BCC8889
CAS No.:101-61-1
- MK-5108 (VX-689)
Catalog No.:BCC2176
CAS No.:1010085-13-8
- Microcystin-LR
Catalog No.:BCC5339
CAS No.:101043-37-2
Selective oxidation of trimethylolpropane to 2,2-bis(hydroxymethyl)butyric acid using growing cells of Corynebacterium sp. ATCC 21245.[Pubmed:26804932]
J Biotechnol. 2016 Mar 10;221:62-9.
Multifunctional chemicals including hydroxycarboxylic acids are gaining increasing interest due to their growing applications in the polymer industry. One approach for their production is a biological selective oxidation of polyols, which is difficult to achieve by conventional chemical catalysis. In the present study, trimethylolpropane (TMP), a trihydric alcohol, was subjected to selective oxidation using growing cells of Corynebacterium sp. ATCC 21245 as a biocatalyst and yielding the dihydroxy-monocarboxylic acid, 2,2-Bis(hydroxymethyl)butyric acid (BHMB). The study revealed that co-substrates are crucial for this reaction. Among the different evaluated co-substrates, a mixture of glucose, xylose and acetate at a ratio of 5:5:2 was found optimum. The optimal conditions for biotransformation were pH 8, 1v/v/m airflow and 500rpm stirring speed. In batch mode of operation, 70.6% of 5g/l TMP was converted to BHMB in 10 days. For recovery of the product the adsorption pattern of BHMB to the anion exchange resin, Ambersep((R)) 900 (OH(-)), was investigated in batch and column experiments giving maximum static and dynamic binding capacities of 135 and 144mg/g resin, respectively. BHMB was separated with 89.7% of recovery yield from the fermentation broth. The approach is applicable for selective oxidation of other highly branched polyols by biotransformation.


