2-Furoyl-LIGRLO-amidePotent and selective PAR2 agonist CAS# 729589-58-6 |
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 729589-58-6 | SDF | Download SDF |
| PubChem ID | 10395438 | Appearance | Powder |
| Formula | C36H63N11O8 | M.Wt | 777.96 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
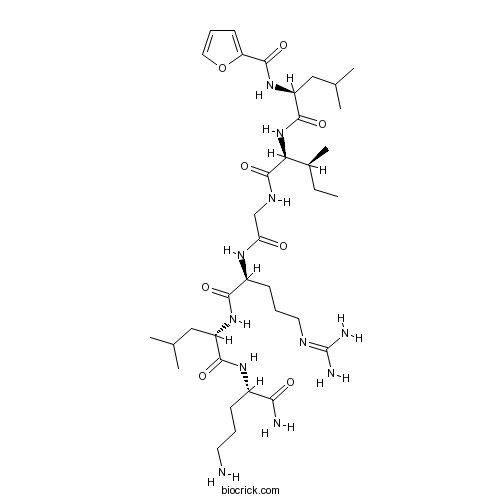
| Sequence | LIGRLX (Modifications: Leu-1 = 2-Furoyl-Leu, X = Orn & C-terminal amide) | ||
| Chemical Name | N-[(2S)-1-[[(2S,3S)-1-[[2-[[(2S)-5-(diaminomethylideneamino)-1-[[(2S)-1-[[(2S)-1,5-diamino-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-3-methyl-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]furan-2-carboxamide | ||
| SMILES | CCC(C)C(C(=O)NCC(=O)NC(CCCN=C(N)N)C(=O)NC(CC(C)C)C(=O)NC(CCCN)C(=O)N)NC(=O)C(CC(C)C)NC(=O)C1=CC=CO1 | ||
| Standard InChIKey | OSKIRYSKGDEIOG-WTWMNNMUSA-N | ||
| Standard InChI | InChI=1S/C36H63N11O8/c1-7-22(6)29(47-33(52)26(18-21(4)5)46-34(53)27-13-10-16-55-27)35(54)42-19-28(48)43-24(12-9-15-41-36(39)40)31(50)45-25(17-20(2)3)32(51)44-23(30(38)49)11-8-14-37/h10,13,16,20-26,29H,7-9,11-12,14-15,17-19,37H2,1-6H3,(H2,38,49)(H,42,54)(H,43,48)(H,44,51)(H,45,50)(H,46,53)(H,47,52)(H4,39,40,41)/t22-,23-,24-,25-,26-,29-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective PAR2 receptor agonist (pD2 = 7.0). Causes a dose-dependent relaxation of murine femoral arteries. |

2-Furoyl-LIGRLO-amide Dilution Calculator

2-Furoyl-LIGRLO-amide Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
2-
- Carvedilol
Catalog No.:BCC4324
CAS No.:72956-09-3
- DL-Catechin
Catalog No.:BCN6325
CAS No.:7295-85-4
- 30-Hydroxylup-20(29)-en-3-one
Catalog No.:BCN4286
CAS No.:72944-06-0
- Octadecyl p-coumarate
Catalog No.:BCN7235
CAS No.:72943-88-5
- K858
Catalog No.:BCC7760
CAS No.:72926-24-0
- Bohemamine
Catalog No.:BCN1958
CAS No.:72926-12-6
- (RS)-4-Carboxyphenylglycine
Catalog No.:BCC6601
CAS No.:7292-81-1
- α,β-Methyleneadenosine 5'-triphosphate trisodium salt
Catalog No.:BCC3591
CAS No.:7292-42-4
- (Z)-Butylidenephthalide
Catalog No.:BCN4007
CAS No.:72917-31-8
- O-Methylcedrelopsin
Catalog No.:BCN3637
CAS No.:72916-61-1
- Isocrocandine
Catalog No.:BCN2071
CAS No.:72903-70-9
- 4-Propenylbrenzcatechin
Catalog No.:BCN3672
CAS No.:72898-29-4
- 6,7-Dihydroneridienone A
Catalog No.:BCN4020
CAS No.:72959-46-7
- Gomisin E
Catalog No.:BCN7031
CAS No.:72960-21-5
- Gomisin O
Catalog No.:BCN2875
CAS No.:72960-22-6
- XRP44X
Catalog No.:BCC7568
CAS No.:729605-21-4
- BMS-707035
Catalog No.:BCC2133
CAS No.:729607-74-3
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
- L-(-)-threo-3-Hydroxyaspartic acid
Catalog No.:BCC6565
CAS No.:7298-99-9
- Cordycepin
Catalog No.:BCN5389
CAS No.:73-03-0
- H-Trp-OH
Catalog No.:BCC3111
CAS No.:73-22-3
- Adenine
Catalog No.:BCC4450
CAS No.:73-24-5
- Melatonin
Catalog No.:BCN2196
CAS No.:73-31-4
- H-Ile-OH
Catalog No.:BCC2960
CAS No.:73-32-5
2-furoyl-LIGRLO-amide: a potent and selective proteinase-activated receptor 2 agonist.[Pubmed:14976230]
J Pharmacol Exp Ther. 2004 Jun;309(3):1124-31.
A peptide corresponding to a proteinase-activated receptor 2 (PAR(2))-activating peptide with an N-terminal furoyl group modification, 2-furoyl-LIGRLO-NH(2), was assessed for PAR(2)-dependent and -independent biological activities. 2-Furoyl-LIGRLO-NH(2) was equally effective to and 10 to 25 times more potent than SLIGRLNH(2) for increasing intracellular calcium in cultured human and rat PAR(2)-expressing cells, respectively. In bioassays of tissue PAR(2) activity, measured as arterial vasodilation and hyperpolarization, 2-furoyl-LIGRLO-NH(2) was 10 to 300 times more potent than SLIGRL-NH(2). Unlike trans-cinnamoyl-LIGRLO-NH(2), 2-furoyl-LI-GRLO-NH(2) did not cause a prominent non-PAR(2)-mediated contraction of murine femoral arteries. In conclusion, 2-furoyl-LI-GRLO-NH(2) represents the most potent and selective activator of PAR(2) in biological systems described to date.
Derivatized 2-furoyl-LIGRLO-amide, a versatile and selective probe for proteinase-activated receptor 2: binding and visualization.[Pubmed:18477767]
J Pharmacol Exp Ther. 2008 Aug;326(2):453-62.
The proteinase-activated receptor-2 (PAR2)-activating peptide with an N-terminal furoyl group modification, 2-furoyl-LIGRLO-NH2 (2fLI), was derivatized via its free ornithine amino group to yield [3H]propionyl-2fLI and Alexa Fluor 594-2fLI that were used as receptor probes for ligand binding assays and receptor visualization both for cultured cells in vitro and for colonic epithelial cells in vivo. The binding of the radiolabeled and fluorescent PAR2 probes was shown to be present in PAR2-transfected Kirsten normal rat kidney cells, but not in vector-alone-transfected cells, and was abolished by pretreatment of cells with saturating concentrations of receptor-selective PAR2 peptide agonists such as SLIGRL-NH2 and the parent agonist 2fLI but not by reverse-sequence peptides such as 2-furoyl-OLRGIL-NH2 that cannot activate PAR2. The relative orders of potencies for a series of PAR2 peptide agonists to compete for the binding of [3H]propionyl-2fLI (2fLI >> SLIGRL-NH2 approximately= trans-cinnamoyl-LIGRLO-NH2 > SLIGKV-NH2 > SLIGKT-NH2) mirrored qualitatively their relative potencies for PAR2-mediated calcium signaling in the same cells or for vasorelaxation in a rat aorta vascular assay. In the vascular assay, the potency of Alexa Fluor 594-2fLI was the same as 2fLI. We conclude that ornithine-derivatized 2fLI peptides are conveniently synthesized PAR2 probes that will be of value for future studies of receptor binding and visualization.
Proteinase-activated receptors 1 and 2 in rat olfactory system: layer-specific regulation of multiple signaling pathways in the main olfactory bulb and induction of neurite retraction in olfactory sensory neurons.[Pubmed:17434682]
Neuroscience. 2007 May 25;146(3):1289-301.
Proteinase-activated receptors (PARs) are a family of four G protein-coupled receptors that are widely distributed in the CNS and involved in neural cell proliferation, differentiation and survival. The olfactory system undergoes continuous neurogenesis throughout life and may represent a critical target of PAR cellular actions. In the present study we investigated the functional activity of PAR1 and PAR2 in microdissected tissue preparations of olfactory nerve-glomerular layer (ON-GL), external plexiform layer (EPL) and granule cell layer (GRL) of the rat main olfactory bulb and in primary cultures of olfactory neuroepithelial cells. Activation of either PAR1 or PAR2 regulated multiple signaling pathways, including activation of pertussis-toxin sensitive Gi/o proteins, inhibition of cyclic AMP formation, stimulation of Gq/11-mediated phosphoinositide (PI) hydrolysis, phosphorylation of Ca2+/calmodulin-dependent protein kinase II and activation of the monomeric G protein Rho, predominantly in ON-GL, whereas only activation of Rho was detected in the deeper layers. Olfactory nerve lesion by nasal irrigation with ZnSO4 induced a marked decrease of PAR signaling in ON-GL. In primary cultures of olfactory neurons, double immunofluorescence analysis showed the localization of PAR1 and PAR2 in cells positive for olfactory-marker protein and neuron-specific enolase. Cell exposure to either nanomolar concentrations of thrombin and trypsin or PAR-activating peptides caused rapid neurite retraction. This study provides the first characterization of the laminar distribution of PAR1 and PAR2 signaling in rat olfactory bulb, demonstrates the presence of the receptors in olfactory sensory neurons and suggests a role of PARs in olfactory sensory neuron neuritogenesis.
A protease activated receptor-2 (PAR-2) activating peptide, tc-LIGRLO-NH2, induces protease release from mast cells: role in TNF degradation.[Pubmed:15265236]
BMC Pharmacol. 2004 Jul 20;4:12.
BACKGROUND: Mast cell (MC)-derived serine proteases have been implicated in a variety of inflammatory processes. We have previously shown that rat peritoneal MC (PMC) express mRNA for protease activated receptor 2 (PAR-2), a G-coupled receptor activated by trypsin-like proteases. Recent evidence also suggests that MC-induced inflammation can be mediated through PAR. Therefore, we hypothesized that specific PAR-2 agonist peptides (PAR-2ap) induce protease release from PMC. RESULTS: Western blot analysis of PMC supernatants revealed that a PAR-2ap, tc-LIGRLO (10 microM), stimulated the release of rat MC protease (RMCP)-1, RMCP-5 and carboxypeptidase-A. The release was evident by 20 min but further increased up to 8 h. To study the biological effects of protease release we tested supernatants from tc-LIGRLO, tc-OLRGIL (inactive control peptide) and antigen-activated PMC for proteolytic activity by seeding with TNF (150 pg/ml), incubating for 8 h at 37 degrees C, and measuring TNF remaining in the supernatants. Supernatants from tc-LIGRLO-stimulated PMC degraded 44 % of seeded TNF (n = 5). Moreover, this TNF proteolysis was dependent on the concentration of tc-LIGRLO used to stimulate PMC, and was significantly inhibited (94 %) by soybean trypsin inhibitor. Antigen and tc-OLRGIL induced no significant release of such proteolytic activity. CONCLUSIONS: These data indicate that a PAR-2ap induces the release of proteases from mast cells, which may degrade extracellular cytokines and other substrates thus modulating the inflammatory response.


