Thrombin Receptor Agonist PeptideCauses platelet aggregation and secretion CAS# 137339-65-2 |
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
- AC 55541
Catalog No.:BCC3951
CAS No.:916170-19-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure
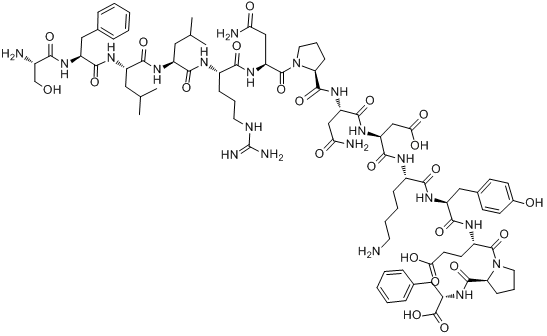
3D structure
| Cas No. | 137339-65-2 | SDF | Download SDF |
| PubChem ID | 16131180 | Appearance | Powder |
| Formula | C81H118N20O23 | M.Wt | 1739.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | TRAP | ||
| Solubility | DMSO : 100 mg/mL (57.47 mM; Need ultrasonic) H2O : ≥ 50 mg/mL (28.74 mM) *"≥" means soluble, but saturation unknown. | ||
| Sequence | SFLLRNPNDKYEPF | ||
| SMILES | CC(C)CC(C(=O)NC(CC(C)C)C(=O)NC(CCCNC(=N)N)C(=O)NC(CC(=O)N)C(=O)N1CCCC1C(=O)NC(CC(=O)N)C(=O)NC(CC(=O)O)C(=O)NC(CCCCN)C(=O)NC(CC2=CC=C(C=C2)O)C(=O)NC(CCC(=O)O)C(=O)N3CCCC3C(=O)NC(CC4=CC=CC=C4)C(=O)O)NC(=O)C(CC5=CC=CC=C5)NC(=O)C(CO)N | ||
| Standard InChIKey | OXHYRVSBKWIFES-WWSDOYNLSA-N | ||
| Standard InChI | InChI=1S/C81H118N20O23/c1-43(2)34-53(93-71(114)54(35-44(3)4)94-73(116)55(92-67(110)49(83)42-102)36-45-16-7-5-8-17-45)70(113)90-51(21-13-31-88-81(86)87)69(112)98-59(40-64(85)105)79(122)101-33-15-22-61(101)76(119)97-57(39-63(84)104)74(117)96-58(41-66(108)109)75(118)89-50(20-11-12-30-82)68(111)95-56(37-47-24-26-48(103)27-25-47)72(115)91-52(28-29-65(106)107)78(121)100-32-14-23-62(100)77(120)99-60(80(123)124)38-46-18-9-6-10-19-46/h5-10,16-19,24-27,43-44,49-62,102-103H,11-15,20-23,28-42,82-83H2,1-4H3,(H2,84,104)(H2,85,105)(H,89,118)(H,90,113)(H,91,115)(H,92,110)(H,93,114)(H,94,116)(H,95,111)(H,96,117)(H,97,119)(H,98,112)(H,99,120)(H,106,107)(H,108,109)(H,123,124)(H4,86,87,88)/t49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Agonist at the thrombin receptor; causes platelet aggregation (EC50 = 4 μM) and secretion. |

Thrombin Receptor Agonist Peptide Dilution Calculator

Thrombin Receptor Agonist Peptide Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Agonist at the thrombin receptor; causes platelet aggregation (EC50 = 4 μM) and secretion.
- LY 215840
Catalog No.:BCC7101
CAS No.:137328-52-0
- Cathepsin S inhibitor
Catalog No.:BCC1455
CAS No.:1373215-15-6
- Dehydroeffusol
Catalog No.:BCN2927
CAS No.:137319-34-7
- 4,5-Dioxo-4,5-seco-11(13)-cadinen-12-oic acid
Catalog No.:BCN1577
CAS No.:137288-61-0
- 4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pymol[2,3-d]pyrimodin-5-yl)ethyl]benzoic acid
Catalog No.:BCC8670
CAS No.:137281-39-1
- Pemetrexed
Catalog No.:BCC9115
CAS No.:137281-23-3
- Boc-Gln-OH
Catalog No.:BCC3382
CAS No.:13726-85-7
- Boc-Glu(OtBu)-OH
Catalog No.:BCC3392
CAS No.:13726-84-6
- Boc-Hyp-OH
Catalog No.:BCC3251
CAS No.:13726-69-7
- Boc-Asp-OH
Catalog No.:BCC2609
CAS No.:13726-67-5
- Chlorantholide E
Catalog No.:BCN4836
CAS No.:1372558-36-5
- Chlorantholide C
Catalog No.:BCN4837
CAS No.:1372558-35-4
- Boc-Lys-OH
Catalog No.:BCC3410
CAS No.:13734-28-6
- Boc-Phe-OH
Catalog No.:BCC3432
CAS No.:13734-34-4
- Boc-Sar-OH
Catalog No.:BCC3337
CAS No.:13734-36-6
- Boc-Ser(tBu)-OH
Catalog No.:BCC3444
CAS No.:13734-38-8
- Boc-Thr(tBu)-OH
Catalog No.:BCC3452
CAS No.:13734-40-2
- Boc-Val-OH
Catalog No.:BCC3465
CAS No.:13734-41-3
- GSK J1
Catalog No.:BCC2231
CAS No.:1373422-53-7
- GSK J4 HCl
Catalog No.:BCC2230
CAS No.:1373423-53-0
- Nω-Propyl-L-arginine hydrochloride
Catalog No.:BCC6965
CAS No.:137361-05-8
- PF-5274857
Catalog No.:BCC3838
CAS No.:1373615-35-0
- Spathulatol
Catalog No.:BCN6877
CAS No.:1373888-27-7
- Diacerein
Catalog No.:BCN2291
CAS No.:13739-02-1
A Double-Chambered Protein Nanocage Loaded with Thrombin Receptor Agonist Peptide (TRAP) and gamma-Carboxyglutamic Acid of Protein C (PC-Gla) for Sepsis Treatment.[Pubmed:26414883]
Adv Mater. 2015 Nov;27(42):6637-43.
New protein nanocages are designed bearing two functional proteins, gamma-carboxyglutamic acid of protein C (PC-Gla) and Thrombin Receptor Agonist Peptide (TRAP), and have an anti-septic response. These nanoparticles reduce sepsis-induced organ injury and septic mortality in vivo. Noting that there are currently no medications for severe sepsis, these results show that novel nanoparticles can be used to treat sepsis.
Thrombin receptor agonist peptide entrapped in poly(D,L)-lactide-co-glycolide microparticles: preparation and characterization.[Pubmed:17454424]
J Microencapsul. 2007 Mar;24(2):129-42.
Thrombin Receptor Agonist Peptide (TRAP-6) could advantageously replace thrombin in terms of accelerating wound healing being less expensive and more stable. To promote TRAP-6 pharmacological action as a tissue reconstruction stimulator this study investigated its entrapment within poly(D,L)-lactide-co-glycolide (PLGA) microparticles. Due to its low molecular weight and water solubility, TRAP-6 microencapsulated form is expected to be more useful. This paper reports TRAP-6 microencapsulation by a double (w/o/w) emulsion-evaporation technique. TRAP-6 release kinetics were evaluated by both chemical (HPLC) and biological assays in vitro. The results revealed a high level of TRAP-6 sensitivity to physico-chemical events during the microencapsulation. The surface morphology difference between control microparticles (without TRAP-6) and microparticles with entrapped TRAP-6 during in vitro degradation highlighted a particular role of TRAP-6. The results can allow one to optimize the microencapsulation procedure and to encounter a new promising approach to development of biodegradable polymer drug delivery systems for wound healing.
Thrombin receptor agonist Peptide immobilized in microspheres stimulates reparative processes in rats with gastric ulcer.[Pubmed:17369897]
Bull Exp Biol Med. 2006 Jul;142(1):35-8.
The effect of synthetic thrombin receptor (PAR1) agonist peptide encapsulated in microspheres made of lactic and glycolic acid copolymer on tissue reparation was studied in rats with acetate-induced ulcer. PAR1 agonist peptide was immobilized in biodegraded lactic and glycolic acid microspheres by double emulgation, the kinetics of peptide release was analyzed, and the dynamics of ulcer healing was studied in experimental (administration of microspheres with the peptide into the stomach) and two control groups (administration of saline or spheres without peptide). Thrombin Receptor Agonist Peptide gradually released from lactic and glycolic acid microspheres into the stomach shortened the inflammation phase and shifted the proliferation phase to the earlier period, thus accelerating healing of experimental ulcers in rats.
Activation of platelet-rich plasma using thrombin receptor agonist peptide.[Pubmed:15789326]
J Oral Maxillofac Surg. 2005 Apr;63(4):529-35.
PURPOSE: This study proposes an alternative preparation method of platelet-rich plasma (PRP). Specifically, we compare the use of Thrombin Receptor Agonist Peptide-6 (TRAP) and bovine thrombin as a clotting agent in the preparation of PRP. MATERIALS AND METHODS: PRP was prepared by centrifugation and clotted with thrombin or TRAP. In vitro clotting times were monitored as a function of TRAP concentration, and clot retraction was determined by measuring clot diameter over time. Following the optimization of TRAP concentration, experiments were repeated with the addition of several commercially available bone substitutes. The release of PRP-relevant growth factors as a function of PRP preparation was also determined. RESULTS: The most rapid polymerization of PRP takes place with the addition of thrombin, followed by TRAP/Allogro (Ceramed, Lakewood, CO), TRAP/BioGlass (Mo-Sci, Rolla, MN), TRAP/BioOss (Osteohealth, Shirley, NY), and TRAP alone. Thrombin caused considerable clot retraction (43%), whereas TRAP alone resulted in only 15% retraction. TRAP/Allogro, TRAP/BioOss, and TRAP/BioGlass all exhibited minimal retraction (8%). CONCLUSIONS: The use of TRAP to activate clot formation in the preparation of PRP may be a safe alternative to bovine thrombin. It results in an excellent working time and significantly less clot retraction than the currently available methods of PRP production.
Structure-function relationships in the activation of platelet thrombin receptors by receptor-derived peptides.[Pubmed:1313429]
J Biol Chem. 1992 Mar 25;267(9):6081-5.
According to present models, thrombin activates platelets by cleaving its receptors after Arg41, creating a new N terminus which acts as a tethered ligand. In support of this model, a peptide (SFLLRNPNDKYEPF or TRP42/55) corresponding to residues 42-55 has been shown to activate the receptor. In the present studies, the structural basis for thrombin receptor activation was examined using fragments of this peptide, as well as variants of the peptide with selected amino acid substitutions. The results show that the features of SFLLRNPNDKYEPF required to mimic the effects of thrombin reside within the first 6 residues, SFLLRN. A hexapeptide comprised of these residues was approximately 5 times more potent than the parent peptide in assays of platelet aggregation and, in addition, caused tyrosine phosphorylation, inhibition of cAMP formation, and an increase in cytosolic Ca2+. Omission of either the Ser residue or the Arg and Asn residues greatly diminished peptide activity, as did the substitution of Ala for Phe or Arg. Substitution of Ala for Ser or the initial Leu, on the other hand, had little adverse effect. The inactive peptides SALLRN and NPNDKYEPF had no effect on platelet activation initiated by SFLLRN, but FLLRN inhibited platelet aggregation in response to both SFLLRN and thrombin. These results suggest that within SFLLRN the Phe and Arg residues are particularly important and that Phe must be preceded by another amino acid, the identity of which is not tightly constrained. This observation and comparisons with the homologous domains of proteins whose tertiary structure is known were used to predict the conformation of the SFLLR sequence. The model which emerged suggests that the SFLLR domain may be part of an extended beta structure in the intact receptor and that cleavage by thrombin causes it to contract and assume a modified helical configuration. In this predicted conformation the side chains of Phe and Arg point in the same direction, potentially into a pocket formed by the remainder of the receptor.
"Thrombin" receptor-directed ligand accounts for activation by thrombin of platelet phospholipase C and accumulation of 3-phosphorylated phosphoinositides.[Pubmed:1655750]
J Biol Chem. 1991 Oct 5;266(28):18435-8.
Using three experimental approaches, we have addressed the questions of whether the presence of saturably bound thrombin plays a role in potentiating the activation of platelet phospholipase C (PLC) and/or accumulation of the 3-phosphorylated phosphoinositides (3-PPI), i.e. phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate, and whether the generation of tethered ligand (Vu, T-K.H., Hung, D. T., Wheaton, V. I., and Coughlin, S. R. (1991) Cell 64, 1057-1068) by thrombin can account fully for thrombin's proteolytic effects in activating platelets, as gauged by the above parameters. We have 1) measured PLC activation or 3-PPI after we have exposed platelets to thrombin for various periods and either blocked thrombin's proteolytic activity without interrupting its binding or blocked both binding and proteolytic activity of thrombin; 2) attempted to potentiate 3-PPI accumulation, using combinations of protein kinase C stimulation, Ca2+ elevation, and saturating but proteolytically inactive thrombins; and 3) compared the activation of platelets by thrombin with activation by the "thrombin" receptor-directed peptide, SFLLRNPNDKYEPF (SFLL; a portion of the tethered ligand created by thrombin's proteolytic activity), and examined the effect of thrombin on this latter activation. We conclude that the initial and sustained effects of thrombin in stimulating PLC and the accumulation of 3-PPI are completely attributable to thrombin's proteolytic activity. Further, thrombin's effects in promoting these responses can be accounted for by the actions of SFLL peptide, and by implication, formation of tethered ligand.
Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation.[Pubmed:1672265]
Cell. 1991 Mar 22;64(6):1057-68.
We isolated a cDNA encoding a functional human thrombin receptor by direct expression cloning in Xenopus oocytes. mRNA encoding this receptor was detected in human platelets and vascular endothelial cells. The deduced amino acid sequence revealed a new member of the seven transmembrane domain receptor family with a large amino-terminal extracellular extension containing a remarkable feature. A putative thrombin cleavage site (LDPR/S) resembling the activation cleavage site in the zymogen protein C (LDPR/I) was noted 41 amino acids carboxyl to the receptor's start methionine. A peptide mimicking the new amino terminus created by cleavage at R41 was a potent agonist for both thrombin receptor activation and platelet activation. "Uncleavable" mutant thrombin receptors failed to respond to thrombin but were responsive to the new amino-terminal peptide. These data reveal a novel signaling mechanism in which thrombin cleaves its receptor's amino-terminal extension to create a new receptor amino terminus that functions as a tethered ligand and activates the receptor.


