A 1120High affinity retinol-binding protein 4 (RBP4) ligand CAS# 1152782-19-8 |
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Sesamolin
Catalog No.:BCN1289
CAS No.:526-07-8
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- Harpagide
Catalog No.:BCN4996
CAS No.:6926-08-5
- Levistilide A
Catalog No.:BCN1197
CAS No.:88182-33-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1152782-19-8 | SDF | Download SDF |
| PubChem ID | 25138295 | Appearance | Powder |
| Formula | C20H19F3N2O3 | M.Wt | 392.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 25 mM in ethanol | ||
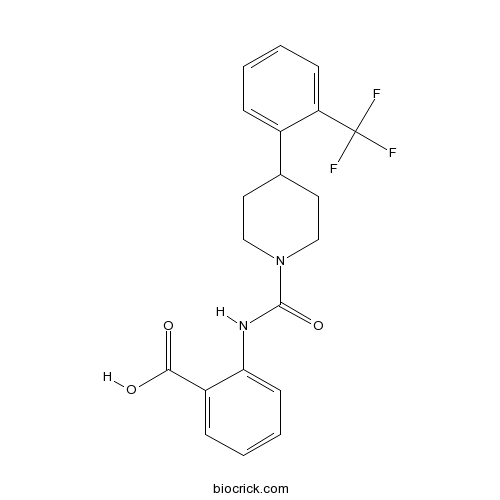
| Chemical Name | 2-[[4-[2-(trifluoromethyl)phenyl]piperidine-1-carbonyl]amino]benzoic acid | ||
| SMILES | C1CN(CCC1C2=CC=CC=C2C(F)(F)F)C(=O)NC3=CC=CC=C3C(=O)O | ||
| Standard InChIKey | MEAQCLPMSVEOQF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H19F3N2O3/c21-20(22,23)16-7-3-1-5-14(16)13-9-11-25(12-10-13)19(28)24-17-8-4-2-6-15(17)18(26)27/h1-8,13H,9-12H2,(H,24,28)(H,26,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity retinol-binding protein 4 (RBP4) ligand (Ki = 8.3 nM); non-retinoid. Selective against a range of different cellular targets. As efficacious as fenretinide in the reduction of serum RBP4 and retinol. Displaces transthyretin (TTR) from RBP4-TTR complexes. | |||||

A 1120 Dilution Calculator

A 1120 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5486 mL | 12.7431 mL | 25.4861 mL | 50.9723 mL | 63.7154 mL |
| 5 mM | 0.5097 mL | 2.5486 mL | 5.0972 mL | 10.1945 mL | 12.7431 mL |
| 10 mM | 0.2549 mL | 1.2743 mL | 2.5486 mL | 5.0972 mL | 6.3715 mL |
| 50 mM | 0.051 mL | 0.2549 mL | 0.5097 mL | 1.0194 mL | 1.2743 mL |
| 100 mM | 0.0255 mL | 0.1274 mL | 0.2549 mL | 0.5097 mL | 0.6372 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dofetilide
Catalog No.:BCC3770
CAS No.:115256-11-6
- VX-661
Catalog No.:BCC1241
CAS No.:1152311-62-0
- 8-pCPT-2-O-Me-cAMP-AM
Catalog No.:BCC6305
CAS No.:1152197-23-3
- Z-Met-OH
Catalog No.:BCC2760
CAS No.:1152-62-1
- Z-Asp-OH
Catalog No.:BCC2793
CAS No.:1152-61-0
- ICI 199,441 hydrochloride
Catalog No.:BCC6792
CAS No.:115199-84-3
- Caprarioside
Catalog No.:BCN7278
CAS No.:1151862-69-9
- 6-Epiharpagoside
Catalog No.:BCN3981
CAS No.:1151862-67-7
- Boc-D-Lys(Fmoc)-OH
Catalog No.:BCC3422
CAS No.:115186-31-7
- Antagonist G
Catalog No.:BCC5858
CAS No.:115150-59-9
- MK 571
Catalog No.:BCC7334
CAS No.:115104-28-4
- MK-571 sodium salt hydrate
Catalog No.:BCC8076
CAS No.:115103-85-0
- 3-Bromo-N-phenylcarbazole
Catalog No.:BCN2260
CAS No.:1153-85-1
- Isosalvipuberulin
Catalog No.:BCN6030
CAS No.:115321-32-9
- Phellochin
Catalog No.:BCN6031
CAS No.:115334-04-8
- Dihydroniloticin
Catalog No.:BCN6032
CAS No.:115334-05-9
- NAN-190 hydrobromide
Catalog No.:BCC6693
CAS No.:115338-32-4
- Siguazodan
Catalog No.:BCC6954
CAS No.:115344-47-3
- Kanshone A
Catalog No.:BCN7279
CAS No.:115356-18-8
- 2,2-Dimethyl-6-phenylpyrano[3,4-b]pyran-8-one
Catalog No.:BCN7280
CAS No.:1153624-36-2
- Kanshone B
Catalog No.:BCN7700
CAS No.:115370-61-1
- EIPA
Catalog No.:BCC7672
CAS No.:1154-25-2
- Molidustat (BAY85-3934)
Catalog No.:BCC6412
CAS No.:1154028-82-6
- Niloticin
Catalog No.:BCN6033
CAS No.:115404-57-4
Efficacy of BIBF 1120 or BIBF 1120 plus chemotherapy on nasopharyngeal carcinoma in vitro and in vivo.[Pubmed:27042009]
Drug Des Devel Ther. 2016 Mar 15;10:1173-80.
PURPOSE: BIBF 1120 is a potent triple angiokinase inhibitor now being evaluated in many types of tumors. We examine the antitumor effects of BIBF 1120 on nasopharyngeal carcinoma (NPC) in vitro and in vivo. MATERIALS AND METHODS: The effect of BIBF 1120 on NPC cell proliferation was evaluated using the Cell Counting Kit 8 assay. The activities of BIBF 1120 as a single agent and in combination with cisplatin (DDP) in NPC tumor xenografts were evaluated by measuring microvessel density and expression of vascular endothelial growth factor signaling. RESULTS: BIBF 1120 exhibited limited inhibition of the growth of three NPC cell lines. Concurrent administration of BIBF 1120 and DDP provided greater antitumor effects compared to that observed with the use of either inhibitor as a single agent in the NPC xenograft model. Microvessel density and expression of vascular endothelial growth factor signaling were significantly reduced. CONCLUSION: BIBF 1120, either as a single agent or in combination with DDP, demonstrates significant antitumor and antiangiogenic effects in the NPC xenograft model. Our results indicate that BIBF 1120 administered in conjunction with chemotherapy might provide an effective treatment method for NPC.
PbTiO3(001) Capped with ZnO(1120): An ab Initio Study of Effect of Substrate Polarization on Interface Composition and CO2 Dissociation.[Pubmed:26996327]
J Phys Chem Lett. 2016 Apr 7;7(7):1310-4.
Catalytic conversion of CO2 into useful chemicals is an attractive alternative to expensive physical carbon sequestration methods. However, this approach is challenging because current chemical conversion methods employ high temperatures or pressures, thereby increasing cost and potentially leading to net carbon positive processes. In this paper, we examine the interface properties of ZnO(1120)/PbTiO3 and its surface interaction with CO2, CO and O. We show that the stoichiometry of the stable interface is dependent on the substrate polarization and can be controlled by changing the growth conditions. Using a model reaction, we demonstrate that a dynamically tuned catalysis scheme could enable significantly lower-energy approaches for CO2 conversion.
Nintedanib (BIBF 1120) blocks the tumor promoting signals of lung fibroblast soluble microenvironment.[Pubmed:27133742]
Lung Cancer. 2016 Jun;96:7-14.
RATIONALE: Nintedanib is a potent, triple angiokinase inhibitor of vascular endothelial growth factor, fibroblast growth factor, and platelet-derived growth factor, and has been recently approved for the treatment of non-small cell lung cancer (NSCLC), following first-line chemotherapy. It is well established that microenvironment plays an important role in tumor progression. Therefore, targeting tumor microenvironment-cancer cell interaction may provide a significant therapeutic target. In this study we tested the effect of Nintedanib on NSCLC cells directly and in the presence of normal and tumor soluble microenvironment. METHODS: Primary fibroblast cultures derived from NSCLC tumors and normal lung tissues were established and their supernatants were collected. These supernatants were added to NSCLC cell lines (H1299, H460 and A549) cultured with/without Nintedanib (0.1-10muM) for 24 and 48h. Cell death (AnnexinV-PI, flow-cytometry), cell number, proliferation (PCNA), protein expression (immunoblotting) and cell migration (scratch test), were tested. Expression of 10 pro-angiogenic cytokines was measured by ELISA-based quantitative array. RESULTS: Tumor and normal supernatants demonstrated similar pro-metastatic effects on the NSCLC phenotype: both elevated cancer cell number, PCNA levels, reduced total and apoptotic cell death and facilitated cell migration. Nintedanib had limited but significant effects on the NSCLC cell number, cell death and migration, but required high doses. However, at lower doses Nintedanib caused cell detachment and elevated integrin-alpha 5 and EGFR levels, both markers of anoikis resistance. This suggests them as possible targets in combination with Nintedanib. Moreover, Nintedanib completely blocked the supernatants ability to facilitate the aggressive cancer cell characteristics. While cytokine array analysis showed no significant changes in FGF, PDGF or VEGF, we found that both supernatants contained high HGF levels, suggesting it as the facilitator of cell migration and proliferation. CONCLUSION: Our results demonstrate that tumor microenvironment-cancer cell interaction is a therapeutic target and should be considered when new drugs are tested.
1120 nm kHz-linewidth single-polarization single-frequency Yb-doped phosphate fiber laser.[Pubmed:28059364]
Opt Express. 2016 Dec 26;24(26):29794-29799.
A spectrally clean kHz-linewidth single-polarization single-frequency distributed Bragg reflector Yb-doped phosphate fiber (YPF) laser at 1120 nm (> 1100 nm) for the first time is demonstrated. By enhancing the reflectivity of output fiber Bragg grating and optimizing the length of YPF to implement the effective ASE suppression and single-longitudinal-mode long-wavelength lasing, a stable output power of over 62 mW is achieved from a 31-mm-long highly YPF with a linewidth of 5.7 kHz. The signal to noise ratio of > 67 dB, the polarization extinction ratio of > 25 dB, and the relative intensity noise of < -150 dB/Hz for the frequencies above 10.0 MHz are obtained in such single-frequency fiber laser. This narrow linewidth fiber laser is an ideal laser source to generate the coherent single-frequency 560 nm light via frequency doubling for biochemical analysis application.
All-trans retinoic acid lowers serum retinol-binding protein 4 concentrations and increases insulin sensitivity in diabetic mice.[Pubmed:20032483]
J Nutr. 2010 Feb;140(2):311-6.
Recent investigations have demonstrated that elevated serum retinol-binding protein 4 (RBP4) secreted from adipose tissue plays a role in the development of systemic insulin resistance, and lowering RBP4 improves insulin sensitivity. These observations provide a rationale for the development of new antidiabetic agents aimed at reducing serum RBP4 concentrations. In this study, we sought to determine whether retinoic acid (RA) administration decreases serum RBP4 and suppresses insulin resistance in diabetic ob/ob mice. All-trans RA [100 mug/(moused) in corn oil] was administered by stomach intubation to a group of ob/ob mice, with the control group receiving the vehicle for 16 d. Body weight and food intake were monitored. Glucose and insulin tolerance tests were performed. We quantified serum RBP4 and retinol by Western blotting and HPLC, respectively. RA treatment reduced body weight (P < 0.05), basal serum glucose (P < 0.001), serum retinol (P < 0.01), and RBP4 (P < 0.05). It improved insulin sensitivity and decreased the retinol:RBP4 ratio (P < 0.05). These studies suggest that RA is an effective antidiabetic agent that could be considered in the treatment of type 2 diabetes.
Identification and characterization of a non-retinoid ligand for retinol-binding protein 4 which lowers serum retinol-binding protein 4 levels in vivo.[Pubmed:19147488]
J Biol Chem. 2009 Mar 20;284(12):7673-80.
Retinol-binding protein 4 (RBP4) transports retinol from the liver to extrahepatic tissues, and RBP4 lowering is reported to improve insulin sensitivity in mice. We have identified A1120, a high affinity (K(i) = 8.3 nm) non-retinoid ligand for RBP4, which disrupts the interaction between RBP4 and its binding partner transthyretin. Analysis of the RBP4-A1120 co-crystal structure reveals that A1120 induces critical conformational changes at the RBP4-transthyretin interface. Administration of A1120 to mice lowers serum RBP4 and retinol levels but, unexpectedly, does not improve insulin sensitivity. In addition, we show that Rpb4(-/-) mice display normal insulin sensitivity and are not protected from high fat diet-induced insulin resistance. We conclude that lowering RBP4 levels does not improve insulin sensitivity in mice. Therefore, RBP4 lowering may not be an effective strategy for treating diabetes.


