Antagonist GBroad spectrum neuropeptide receptor antagonist CAS# 115150-59-9 |
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Cefoselis
Catalog No.:BCC4092
CAS No.:122841-10-5
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 115150-59-9 | SDF | Download SDF |
| PubChem ID | 163960 | Appearance | Powder |
| Formula | C49H66N12O6S | M.Wt | 951.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
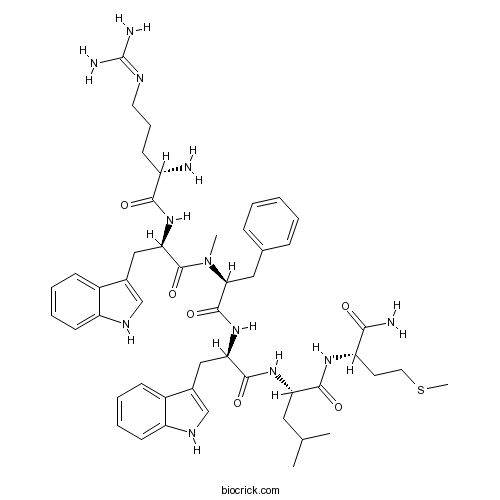
| Synonyms | [Arg<sup>6</sup>,D-Trp<sup>7,9</sup>,<em>N</em>-MePhe<sup>8</sup>]-Substance P(6-11) | ||
| Solubility | Soluble to 1 mg/ml in water | ||
| Sequence | RWFWLM (Modifications: Trp-2 = D-Trp, Phe-3 = N-Methyl-Phe, Trp-5 = D-Trp, Met-7 = C-terminal amide) | ||
| Chemical Name | (2S)-2-[[(2R)-2-[[(2S)-2-[[(2R)-2-[[(2S)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]-3-(1H-indol-3-yl)propanoyl]-methylamino]-3-phenylpropanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-N-[(2S)-1-amino-4-methylsulfanyl-1-oxobutan-2-yl]-4-methylpentanamide | ||
| SMILES | CC(C)CC(C(=O)NC(CCSC)C(=O)N)NC(=O)C(CC1=CNC2=CC=CC=C21)NC(=O)C(CC3=CC=CC=C3)N(C)C(=O)C(CC4=CNC5=CC=CC=C54)NC(=O)C(CCCN=C(N)N)N | ||
| Standard InChIKey | CUCSSYAUKKIDJV-FAXBSAIASA-N | ||
| Standard InChI | InChI=1S/C49H66N12O6S/c1-29(2)23-39(45(64)57-38(43(51)62)20-22-68-4)58-46(65)40(25-31-27-55-36-18-10-8-15-33(31)36)59-47(66)42(24-30-13-6-5-7-14-30)61(3)48(67)41(26-32-28-56-37-19-11-9-16-34(32)37)60-44(63)35(50)17-12-21-54-49(52)53/h5-11,13-16,18-19,27-29,35,38-42,55-56H,12,17,20-26,50H2,1-4H3,(H2,51,62)(H,57,64)(H,58,65)(H,59,66)(H,60,63)(H4,52,53,54)/t35-,38-,39-,40+,41+,42-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Substance P analog that is a broad spectrum neuropeptide antagonist and antiproliferative agent. Blocks Swiss 3T3 cell growth induced by vasopressin, gastrin-releasing peptide and bradykinin. Inhibits neuropeptide-dependent and -independent proliferation of small cell lung cancer in vitro; activates JNK and stimulates apoptosis. Inhibits growth of SCLC xenografts in mice in vivo. |

Antagonist G Dilution Calculator

Antagonist G Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MK 571
Catalog No.:BCC7334
CAS No.:115104-28-4
- MK-571 sodium salt hydrate
Catalog No.:BCC8076
CAS No.:115103-85-0
- Tiagabine
Catalog No.:BCC5243
CAS No.:115103-54-3
- Carmoxirole hydrochloride
Catalog No.:BCC7278
CAS No.:115092-85-8
- Soyacerebroside II
Catalog No.:BCN6029
CAS No.:115074-93-6
- CNQX
Catalog No.:BCC6569
CAS No.:115066-14-3
- Desmethylxanthohumol
Catalog No.:BCN2997
CAS No.:115063-39-3
- 29-Norcycloart-23-ene-3,25-diol
Catalog No.:BCN4727
CAS No.:115040-04-5
- Pseudolaric acid D
Catalog No.:BCN6028
CAS No.:115028-67-6
- SR 16584
Catalog No.:BCC6176
CAS No.:1150153-86-8
- Icariside F2
Catalog No.:BCN6435
CAS No.:115009-57-9
- Cyclo(L-Leu-trans-4-hydroxy-L-Pro)
Catalog No.:BCN3994
CAS No.:115006-86-5
- Boc-D-Lys(Fmoc)-OH
Catalog No.:BCC3422
CAS No.:115186-31-7
- 6-Epiharpagoside
Catalog No.:BCN3981
CAS No.:1151862-67-7
- Caprarioside
Catalog No.:BCN7278
CAS No.:1151862-69-9
- ICI 199,441 hydrochloride
Catalog No.:BCC6792
CAS No.:115199-84-3
- Z-Asp-OH
Catalog No.:BCC2793
CAS No.:1152-61-0
- Z-Met-OH
Catalog No.:BCC2760
CAS No.:1152-62-1
- 8-pCPT-2-O-Me-cAMP-AM
Catalog No.:BCC6305
CAS No.:1152197-23-3
- VX-661
Catalog No.:BCC1241
CAS No.:1152311-62-0
- Dofetilide
Catalog No.:BCC3770
CAS No.:115256-11-6
- A 1120
Catalog No.:BCC7775
CAS No.:1152782-19-8
- 3-Bromo-N-phenylcarbazole
Catalog No.:BCN2260
CAS No.:1153-85-1
- Isosalvipuberulin
Catalog No.:BCN6030
CAS No.:115321-32-9
Dihydromunduletone Is a Small-Molecule Selective Adhesion G Protein-Coupled Receptor Antagonist.[Pubmed:27338081]
Mol Pharmacol. 2016 Sep;90(3):214-24.
Adhesion G protein-coupled receptors (aGPCRs) have emerging roles in development and tissue maintenance and is the most prevalent GPCR subclass mutated in human cancers, but to date, no drugs have been developed to target them in any disease. aGPCR extracellular domains contain a conserved subdomain that mediates self-cleavage proximal to the start of the 7-transmembrane domain (7TM). The two receptor protomers, extracellular domain and amino terminal fragment (NTF), and the 7TM or C-terminal fragment remain noncovalently bound at the plasma membrane in a low-activity state. We recently demonstrated that NTF dissociation liberates the 7TM N-terminal stalk, which acts as a tethered-peptide agonist permitting receptor-dependent heterotrimeric G protein activation. In many cases, natural aGPCR ligands are extracellular matrix proteins that dissociate the NTF to reveal the tethered agonist. Given the perceived difficulty in modifying extracellular matrix proteins to create aGPCR probes, we developed a serum response element (SRE)-luciferase-based screening approach to identify GPR56/ADGRG1 small-molecule inhibitors. A 2000-compound library comprising known drugs and natural products was screened for GPR56-dependent SRE activation inhibitors that did not inhibit constitutively active Galpha13-dependent SRE activation. Dihydromunduletone (DHM), a rotenoid derivative, was validated using cell-free aGPCR/heterotrimeric G protein guanosine 5'-3-O-(thio)triphosphate binding reconstitution assays. DHM inhibited GPR56 and GPR114/ADGRG5, which have similar tethered agonists, but not the aGPCR GPR110/ADGRF1, M3 muscarinic acetylcholine, or beta2 adrenergic GPCRs. DHM inhibited tethered peptide agonist-stimulated and synthetic peptide agonist-stimulated GPR56 but did not inhibit basal activity, demonstrating that it antagonizes the peptide agonist. DHM is a novel aGPCR antagonist and potentially useful chemical probe that may be developed as a future aGPCR therapeutic.
The impact of P2X7 receptor antagonist, brilliant blue G on graft-versus-host disease in mice after allogeneic hematopoietic stem cell transplantation.[Pubmed:27544305]
Cell Immunol. 2016 Dec;310:71-77.
The purpose of this study was to investigate the role of P2X7 on liver inflammation in mice after HSCT. Hematopoietic stem cells obtained from C57BL/6 mice were administrated into BALB/c mice to establish GVHD model. On day 7, 14, 21 and 28 after HSCT, mice received P2X7R antagonist brilliant blue G (BBG) or not were sacrificed for analysis of weight loss, liver inflammation, cytokine secretion, P2X7, NLRP3 expression as well as caspase-1 activation. Liver inflammation with neutrophils and macrophases infiltration as well as weight loss increase was present after HSCT, but improved after administration with high dose of BBG compared with lower dose. High dose of P2X7R inhibitor administration after HSCT previously reduced levels of IL-1beta, IL-18, caspase-1, NLRP3 as well as P2X7, and the level of alanine transaminase (ALT) and the ratio of aspartate amino transferase (AST)/ALT compared with that receiving low dose of BBG. Meanwhile, P2X7R blockage also reduced infiltration of macrophages and neutrophils and levels of CXCL8 and CCL2 in peripheral blood as well as improved liver function. In conclusion, blockage of P2X7R by BBG exerts a protective effect on GVHD post HSCT and improves liver function suggesting that this receptor could be considered as an attractive target for treatment of GVHD.
Identification of the Clinical Candidate (R)-(1-(4-Fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexah ydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methano ne (CORT125134): A Selective Glucocorticoid Receptor (GR) Antagonist.[Pubmed:28368581]
J Med Chem. 2017 Apr 27;60(8):3405-3421.
The nonselective glucocorticoid receptor (GR) antagonist mifepristone has been approved in the U.S. for the treatment of selected patients with Cushing's syndrome. While this drug is highly effective, lack of selectivity for GR leads to unwanted side effects in some patients. Optimization of the previously described fused azadecalin series of selective GR antagonists led to the identification of CORT125134, which is currently being evaluated in a phase 2 clinical study in patients with Cushing's syndrome.
Inhibiting purinergic P2X7 receptors with the antagonist brilliant blue G is neuroprotective in an intranigral lipopolysaccharide animal model of Parkinson's disease.[Pubmed:28035410]
Mol Med Rep. 2017 Feb;15(2):768-776.
Parkinson's disease (PD) is a common neurodegenerative disorder, which is characterized by the selective and progressive death of dopaminergic (DA) neurons in the substantia nigra. Increasing evidence suggests that inflammation is important in the degeneration of DA neurons. The purinergic receptor subtype P2X7 receptor (P2X7R) is key in the activation and proliferation of microglia. The present study aimed to examine whether inhibiting purinergic P2X7 receptors is neuroprotective in a rat model of PD, specifically via inhibiting p38 mitogenactivated protein kinase (MAPK). In an intranigral lipopolysaccharide (LPS) rat model of PD, immunohistochemical analysis revealed enhanced expression of P2X7R was observed in microglia. The administration of the P2X7R antagonist, brilliant blue G (BBG), reduced activation of the microglia and the loss of nigral DA neurons. In addition, immunohistochemistry and western blot analysis revealed the phosphorylation level of p38 MAPK increased in the microglia of the LPSinjected rats, which was inhibited by BBG treatment. The p38 MAPK inhibitor, SB203580, reduced microglial activation and the loss of DA neurons. Thus, these findings suggested that inhibition of P2X7R by BBG attenuated microglial activation and the loss of substantia nigra DA neurons via p38 MAPK in the rat LPS model of PD.
[Arg(6), D-Trp(7,9), N(me)Phe(8)]-substance P (6-11) (antagonist G) induces AP-1 transcription and sensitizes cells to chemotherapy.[Pubmed:10970698]
Br J Cancer. 2000 Oct;83(7):941-8.
[Arg(6), D-Trp(7,9), N(me)Phe(8)]-substance P (6-11) (Antagonist G) inhibits small cell lung cancer (SCLC) growth and is entering Phase II clinical investigation for the treatment of SCLC. As well as acting as a neuropeptide receptor antagonist, Antagonist G stimulates c-jun-N-terminal kinase (JNK) activity and apoptosis in SCLC cells. We extend these findings and show that the stimulation of JNK and apoptosis by Antagonist G is dependent upon the generation of reactive oxygen species (ROS) being inhibited either by anoxia or the presence of N-acetyl cysteine (n-AC). Antagonist G is not intrinsically a free radical oxygen donor but stimulates free radical generation specifically within SCLC cells (6.2-fold) and increases the activity of the redox-sensitive transcription factor AP-1 by 61%. In keeping with this, Antagonist G reduces cellular glutathione (GSH) levels (38% reduction) and stimulates ceramide production and lipid peroxidation (112% increase). At plasma concentrations achieved clinically in the phase I studies, Antagonist G augments, more than additively, growth inhibition induced by etoposide. Our results suggest that Antagonist G may be particularly effective as an additional treatment with standard chemotherapy in SCLC. These novel findings will be important for the clinical application of this new and exciting compound and for the future drug development of new agents to treat this aggressive cancer.
Broad spectrum neuropeptide antagonists inhibit the growth of small cell lung cancer in vivo.[Pubmed:1379515]
Cancer Res. 1992 Aug 15;52(16):4554-7.
The proliferation of small cell lung cancer (SCLC) cells appears sustained by multiple autocrine and paracrine circuits involving Ca2+ mobilizing neuropeptides. Consequently, broad spectrum neuropeptide antagonists which inhibit SCLC growth in vitro have been suggested as potential anticancer agents. Here we evaluated this hypothesis using xenografts of WX322 cells, a SCLC cell line that responds to multiple Ca2+ mobilizing neuropeptides. The broad spectrum neuropeptide antagonists [Arg6,D-Trp7,9,MePhe8]substance P(6-11) and [D-Arg1,D-Phe5,Trp7,9Leu11[substance P were shown to inhibit the growth of WX322 xenografts in nude mice. Similar results were obtained with xenografts of the SCLC cell line H69. The results indicate that broad spectrum neuropeptide antagonists can inhibit the growth of SCLC in vivo and suggest that these antagonists could be useful in the treatment of SCLC.
A neuropeptide antagonist that inhibits the growth of small cell lung cancer in vitro.[Pubmed:1693879]
Cancer Res. 1990 Jul 1;50(13):3968-73.
In the search for novel antiproliferative agents for small cell lung cancer (SCLC), we found the neuropeptide antagonist [Arg6, D-Trp7,9,MePhe8]substance P(6-11) to be effective in vitro. In murine Swiss 3T3 cells [Arg6,D-Trp7,9,MePhe8]substance P(6-11) was identified as a potent inhibitor of vasopressin-stimulated DNA synthesis which also blocks [3H]vasopressin binding to specific cell-surface receptors. It was a less potent antagonist of gastrin-releasing peptide and bradykinin in these cells but did not block the effects of other mitogens. In SCLC cell lines, [Arg6,D-Trp7,9,MePhe8]substance P(6-11) inhibited colony-formation in soft agarose and growth in liquid culture in a dose-dependent manner. It also blocked receptor-mediated Ca2+ mobilization induced by vasopressin, bradykinin, cholecystokinin, galanin, gastrin-releasing peptide, and neurotensin. We suggest that broad-spectrum neuropeptide antagonists can block multiple autocrine and paracrine growth loops in SCLC and could be useful therapeutic agents.


