AGN 192403 hydrochlorideI1 imidazoline receptor CAS# 1021868-90-5 |
- AGI-5198
Catalog No.:BCC2293
CAS No.:1355326-35-0
- Mutant IDH1 inhibitor
Catalog No.:BCC4144
CAS No.:1429180-08-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1021868-90-5 | SDF | Download SDF |
| PubChem ID | 11957452 | Appearance | Powder |
| Formula | C10H20ClN | M.Wt | 189.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
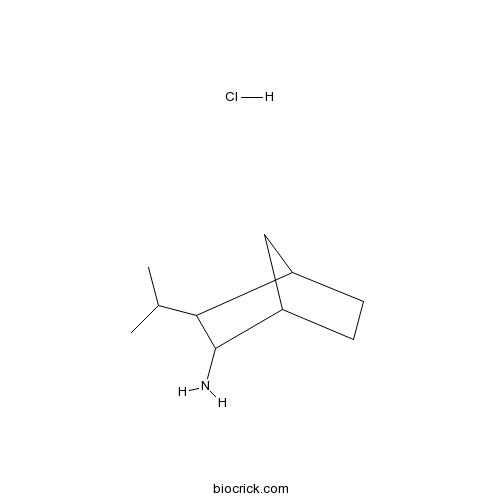
| Chemical Name | 2-propan-2-ylbicyclo[2.2.1]heptan-3-amine;hydrochloride | ||
| SMILES | CC(C)C1C2CCC(C2)C1N.Cl | ||
| Standard InChIKey | KSTGYMXUFHCTSM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H19N.ClH/c1-6(2)9-7-3-4-8(5-7)10(9)11;/h6-10H,3-5,11H2,1-2H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | I1 imidazoline binding site selective ligand with a potency at I1 comparable to moxonidine, but devoid of affinity for adrenoceptors and the I2 binding site. Interestingly, in animal models, this compound causes none of the physiological responses associated with the I1 binding site. |

AGN 192403 hydrochloride Dilution Calculator

AGN 192403 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2706 mL | 26.3532 mL | 52.7065 mL | 105.413 mL | 131.7662 mL |
| 5 mM | 1.0541 mL | 5.2706 mL | 10.5413 mL | 21.0826 mL | 26.3532 mL |
| 10 mM | 0.5271 mL | 2.6353 mL | 5.2706 mL | 10.5413 mL | 13.1766 mL |
| 50 mM | 0.1054 mL | 0.5271 mL | 1.0541 mL | 2.1083 mL | 2.6353 mL |
| 100 mM | 0.0527 mL | 0.2635 mL | 0.5271 mL | 1.0541 mL | 1.3177 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AGN 192403 hydrochloride is a selective ligand of imidazoline1 receptor [1].
Imidazoline receptor is the primary receptor for clonidine and other imidazolines. Imidazoline1 receptor (I1 receptor) lowers blood pressure via inhibition of the sympatho actions by imidazolines.
AGN 192403 hydrochloride is a selective ligand of I1 receptor. AGN192403 exhibited affinity for I1 receptor with Ki value of 42 nM and was only 5-fold less potent than clonidine. However, AGN192403 had no agonist and antagonist activities. In monkey and rat, AGN192403 (5000 μg/kg) had no effect on blood pressure [1]. In astrocytes, AGN 192403 inhibited cytochrome c release, lysosomal acridine orange relocation, caspase-9 activation and decrease in mitochondrial potential, cytotoxicities that was induced by naphthazarin. Also, AGN 192403 inhibited mitochondrial dysfunction and cytotoxicities induced by antimycin A and rotenone, inhibitors of mitochondrial respiration [2]. In isolated normotensive rat hearts, AGN192403 inhibited atrial natriuretic peptide (ANP) release induced by moxonidine (10−6 M) [3].
References:
[1] unk SA1, Lai RK, Burke JE,Munk SA1, Lai RK, Burke JE,Synthesis and pharmacologic evaluation of 2-endo-amino-3-exo-isopropylbicyclo[2.2.1]heptane: a potent imidazoline1 receptor specific agent.Munk et al (1996) Synthesis and pharmacologic evaluation of 2-endo-amino-3-exo-isopropylbicyclo[2.2.1]heptane: a potent imidazoline1 receptor specific agent. J.Med.Chem. 39 1193. PMID: 8632424.Stephen A. Munk,* Ronald K. Lai, James E. Burke, [1]. Munk SA, Lai RK, Burke JE, et al. Synthesis and Pharmacologic Evaluation of 2-endo-Amino-3-exoisopropylbicyclo[2.2.1]heptane: A Potent Imidazoline1 Receptor Specific Agent. J Med Chem, 1996, 39(6): 1193-1195.
[2]. Choi DH, Kim DH, Park YG, et al. Protective effects of rilmenidine and AGN 192403 on oxidative cytotoxicity and mitochondrial inhibitor-induced cytotoxicity in astrocytes. Free Radic Biol Med, 2002, 33(10): 1321-1333.
[3]. Mukaddam-Daher S, Menaouar A, Gutkowska J. Receptors involved in moxonidine-stimulated atrial natriuretic peptide release from isolated normotensive rat hearts. Eur J Pharmacol, 2006, 541(1-2): 73-79.
- ARL 67156 trisodium salt
Catalog No.:BCC7004
CAS No.:1021868-83-6
- PPNDS
Catalog No.:BCC7015
CAS No.:1021868-77-8
- Boc-D-N-Me-Phe.DCHA
Catalog No.:BCC3347
CAS No.:102185-45-5
- Boc-Arg(Mts)-OH
Catalog No.:BCC3054
CAS No.:102185-38-6
- Boc-D-Pro-OSu
Catalog No.:BCC3438
CAS No.:102185-34-2
- DPCPX
Catalog No.:BCC6649
CAS No.:102146-07-6
- RJR 2429 dihydrochloride
Catalog No.:BCC7000
CAS No.:1021418-53-0
- Neoprocurcumenol
Catalog No.:BCN3694
CAS No.:102130-91-6
- Isoprocurcumenol
Catalog No.:BCN3528
CAS No.:102130-90-5
- Atractylic acid dipotassium salt
Catalog No.:BCN5384
CAS No.:102130-43-8
- AM580
Catalog No.:BCC5373
CAS No.:102121-60-8
- rac-Rotigotine Hydrochloride
Catalog No.:BCC1881
CAS No.:102120-99-0
- ZM 39923 HCl
Catalog No.:BCC2203
CAS No.:1021868-92-7
- 3-O-Methyltirotundin
Catalog No.:BCN5837
CAS No.:1021945-29-8
- Boc-4-oxo-Pro-OMe
Catalog No.:BCC3436
CAS No.:102195-80-2
- Tyrosine kinase inhibitor
Catalog No.:BCC2020
CAS No.:1021950-26-4
- ARN2966
Catalog No.:BCC8074
CAS No.:102212-26-0
- Btk inhibitor 1 R enantiomer
Catalog No.:BCC5125
CAS No.:1022150-12-4
- SGX-523
Catalog No.:BCC1055
CAS No.:1022150-57-7
- Epoxyparvinolide
Catalog No.:BCN5838
CAS No.:102227-61-2
- (R)-Eriodictyol-8-C-beta-D-glucopyranoside
Catalog No.:BCN8028
CAS No.:1023271-51-3
- Cadensin D
Catalog No.:BCN7260
CAS No.:102349-35-9
- Naringin
Catalog No.:BCN6312
CAS No.:10236-47-2
- Negsehisandrin G
Catalog No.:BCN2674
CAS No.:1023744-69-5
Receptors involved in moxonidine-stimulated atrial natriuretic peptide release from isolated normotensive rat hearts.[Pubmed:16774751]
Eur J Pharmacol. 2006 Jul 10;541(1-2):73-9.
Imidazoline I1-receptors are present in the heart and may be involved in atrial natriuretic peptide (ANP) release. The following studies investigated whether moxonidine (an antihypertensive imidazoline I1-receptor and alpha2-adrenoceptor agonist) acts directly on the heart to stimulate ANP release, and to characterize the receptor type involved in this action. Perfusion of rat (200-225 g) isolated hearts with moxonidine (10(-6) and 10(-5) M), for 30 min, resulted in ANP release (83+/-29 and 277+/-70 ng/30 min, above basal, respectively), significantly (P<0.01) different from perfusion with buffer (-6+/-31 ng/30 min). ANP release stimulated by moxonidine (10(-6) M) was inhibited by co-perfusion with the antagonists, AGN192403 (imidazoline I1-receptor), phenoxybenzamine (alpha2>alpha1-adrenoceptors), and prazosin (alpha1>alpha2-adrenoceptors), but increased by rauwolscine (alpha2-adrenoceptors). Perfusion with 10(-5) M brimonidine (full alpha2-adrenoceptor agonist) inhibited moxonidine-stimulated ANP release. Similarly, moxonidine (10(-6) M) tended to reduce coronary flow, but significantly increased coronary flow in the presence of brimonidine, which was vasoconstrictive when perfused alone. Coronary flow was reduced by 10(-5) M each, brimonidine>clonidine>moxonidine; while similar bradycardia was observed with clonidine and moxonidine, but not with brimonidine. In conclusion, these results argue in favor of moxonidine acting primarily on imidazoline I1-receptors to release ANP, with both alpha2-adrenoceptor and imidazoline I1-receptors exerting inhibitory inter-relation. In contrast, the coronary vasodilatory effect of moxonidine requires full activation of alpha2-adrenoceptor. The sympatholytic and ANP-releasing effects of moxonidine appear to be mediated by cardiac imidazoline receptors that may be differentially localized. Most importantly, moxonidine can stimulate ANP release from the heart without contribution of the central nervous system.
Agmatine, an endogenous ligand at imidazoline binding sites, does not antagonize the clonidine-mediated blood pressure reaction.[Pubmed:11834614]
Br J Pharmacol. 2002 Feb;135(3):663-72.
Since agmatine has been identified as a clonidine displacing substance (CDS), the aim of this study was to investigate whether agmatine can mimic CDS-induced cardiovascular reactions in organ bath experiments, pithed spontaneously hypertensive rats (SHR) and anaesthetized SHR. Intravenously-administered agmatine significantly reduced the blood pressure and heart rate of anaesthetized SHR at doses higher than 1 and 3 mg kg(-1), respectively. These effects are probably mediated via central mechanisms, since there was an approximate 8 fold rightward shift of the dose-response curve in the pithed SHR (indicating a weakened cardiovascular effect). Moreover, in organ bath experiments, agmatine failed to alter the contractility of intact or endothelium-denuded aortal rings. When agmatine was administered i.c.v. to anaesthetized SHR, blood pressure was increased without any alteration of heart rate, whereas blood pressure was unchanged and heart rate was increased after injection into the 4th brain ventricle. This suggests that haemodynamic reaction patterns after central application are related to distinct influences on central cardiovascular mechanisms. Agmatine reduces noradrenaline release in pithed SHR while alpha(2)-adrenoceptors are irreversibly blocked with phenoxybenzamine, but not while I(1)-binding sites are selectively blocked with AGN192403. This suggests that agmatine may modulate noradrenaline release in the same way that clonidine does, i.e. via imidazoline binding sites; this involves a reduction in sympathetic tone which in turn reduces blood pressure and heart rate. Finally, CDS-like cardiovascular activity appears not to be due to agmatine, since (i) blood pressure in anaesthetized SHR is decreased by agmatine and clonidine, and (ii) agmatine did not antagonize the blood pressure reaction to clonidine in pithed or anaesthetized SHR.


