AM095Potent LPA1 receptor antagonist CAS# 1345614-59-6 |
- AM966
Catalog No.:BCC1355
CAS No.:1228690-19-4
- AM-095 free base
Catalog No.:BCC1352
CAS No.:1228690-36-5
- Ki16198
Catalog No.:BCC4560
CAS No.:355025-13-7
- Ki16425
Catalog No.:BCC1155
CAS No.:355025-24-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1345614-59-6 | SDF | Download SDF |
| PubChem ID | 53303875 | Appearance | Powder |
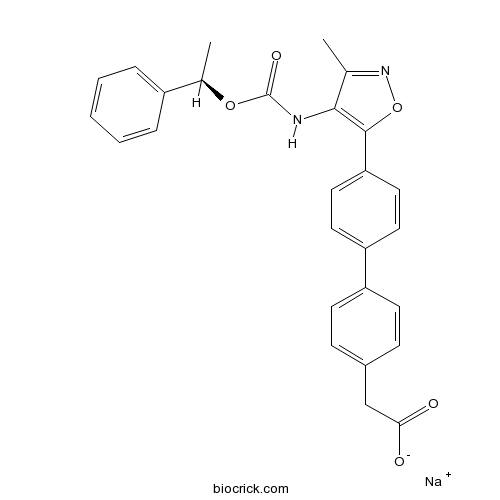
| Formula | C27H23N2NaO5 | M.Wt | 478.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 83.33 mg/mL (174.16 mM; Need ultrasonic) | ||
| Chemical Name | sodium;2-[4-[4-[3-methyl-4-[[(1R)-1-phenylethoxy]carbonylamino]-1,2-oxazol-5-yl]phenyl]phenyl]acetate | ||
| SMILES | CC1=NOC(=C1NC(=O)OC(C)C2=CC=CC=C2)C3=CC=C(C=C3)C4=CC=C(C=C4)CC(=O)[O-].[Na+] | ||
| Standard InChIKey | BDKDADFSIDCQGB-GMUIIQOCSA-M | ||
| Standard InChI | InChI=1S/C27H24N2O5.Na/c1-17-25(28-27(32)33-18(2)20-6-4-3-5-7-20)26(34-29-17)23-14-12-22(13-15-23)21-10-8-19(9-11-21)16-24(30)31;/h3-15,18H,16H2,1-2H3,(H,28,32)(H,30,31);/q;+1/p-1/t18-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AM095 is a potent antagonist of LPA1 receptor with IC50 values of 0.98 and 0.73 μM for recombinant human and mouse LPA1, respectively. | |||||
| Targets | LPA1 receptor | |||||
| IC50 | 0.98 μM (recombinant human) 0.73 μM (recombinant mouse) | |||||
| Cell experiment: [1] | |
| Cell lines | MDA-MB-231 cells and SK-OV3 cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 500 nM, 5 min |
| Applications | Cells were pretreated with AM-095 or vehicle for 5 min and then treated with 10 μM of LPE or LPA. AM-095 (500 nM) completely inhibited LPA-induced [Ca2+]i responses in both cell lines and LPE-induced [Ca2+]i responses in MDA-MB-231 cells. AM-095 (500 nM) did not affect LPE-induced [Ca2+] i responses in SK-OV3 cells. |
| Animal experiment : [2] | |
| Animal models | Female CD-1 mice |
| Dosage form | Oral administration, 1–30 mg/kg |
| Application | Mice received AM095 in a volume of 10 ml/kg 2 h before the intravenous LPA (300 μg/mouse) challenge. LPA stimulated histamine release in a dose-dependent manner, resulting in a nearly 14-fold stimulation at the highest concentration tested. AM095 dose-dependently inhibited histamine release with an ED50 of 8.3 mg/kg and a maximal reduction of 80% at a dose of 30 mg/kg. By plotting the percentage inhibition of histamine release versus AM095 plasma concentrations for each individual animal and assuming a maximum response of 80% we generated an EC50 of ~ 1.5 μM. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Park S J, Lee K P, Im D S. Action and Signaling of Lysophosphatidylethanolamine in MDA-MB-231 Breast Cancer Cells. Biomolecules & therapeutics, 2014, 22(2): 129. [2] Swaney J S, Chapman C, Correa L D, et al. Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor-selective antagonist. Journal of Pharmacology and Experimental Therapeutics, 2011, 336(3): 693-700. | |

AM095 Dilution Calculator

AM095 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.09 mL | 10.45 mL | 20.9 mL | 41.7999 mL | 52.2499 mL |
| 5 mM | 0.418 mL | 2.09 mL | 4.18 mL | 8.36 mL | 10.45 mL |
| 10 mM | 0.209 mL | 1.045 mL | 2.09 mL | 4.18 mL | 5.225 mL |
| 50 mM | 0.0418 mL | 0.209 mL | 0.418 mL | 0.836 mL | 1.045 mL |
| 100 mM | 0.0209 mL | 0.1045 mL | 0.209 mL | 0.418 mL | 0.5225 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AM095 is a novel, potent and orally bioavailable antagonist of lysophosphatidic acid type 1 receptor (LPA1) with IC50 values of 0.73 and 0.98 μM for mouse or recombinant human LPA1, respectively [1].
In vitro, AM095 has shown to inhibit LPA1-induced chemotaxis of both mouse LPA1/CHO cells and human A2058 melanoma cells with IC50 values of 0.78 μM and 0.23μM [1].
In vivo, AM095 could dose-dependently block LPA-induced histamine release with an ED50 value of 8.3 mg/kg in mice. Additionally, AM095 has been revealed to remarkably reduce the BALF collagen and protein with an ED50 value of 10 mg/kg in lungs. AM095 has also shown to decrease both macrophage and lymphocyte infiltration induced by bleomycin in mice [1].
References:
[1] Swaney JS1, Chapman C, Correa LD, Stebbins KJ, Broadhead AR, Bain G, Santini AM, Darlington J, King CD, Baccei CS, Lee C, Parr TA, Roppe JR, Seiders TJ, Ziff J, Prasit P, Hutchinson JH, Evans JF, Lorrain DS. Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor-selective antagonist. J Pharmacol Exp Ther. 2011 Mar;336(3):693-700.
- Atorvastatin Calcium
Catalog No.:BCC2319
CAS No.:134523-03-8
- U 90042
Catalog No.:BCC7465
CAS No.:134516-99-7
- Gardenoin J
Catalog No.:BCN7666
CAS No.:1345109-46-7
- 1-Cinnamoyl-3-hydroxypyrrolidine
Catalog No.:BCN6497
CAS No.:1344876-77-2
- Trimethylvinylammonium(1+)
Catalog No.:BCN1820
CAS No.:13448-18-5
- Richenoic acid
Catalog No.:BCN6185
CAS No.:134476-74-7
- BW-B 70C
Catalog No.:BCC7013
CAS No.:134470-38-5
- Discodermide
Catalog No.:BCN1834
CAS No.:134458-00-7
- CA 074
Catalog No.:BCC1141
CAS No.:134448-10-5
- Dehydroandrographolide
Catalog No.:BCN1260
CAS No.:134418-28-3
- Seocalcitol
Catalog No.:BCC1944
CAS No.:134404-52-7
- Epoxomicin
Catalog No.:BCC1235
CAS No.:134381-21-8
- ETP-46464
Catalog No.:BCC3913
CAS No.:1345675-02-6
- Arginase inhibitor 1
Catalog No.:BCC4034
CAS No.:1345808-25-4
- Altiratinib
Catalog No.:BCC6385
CAS No.:1345847-93-9
- CYM 50308
Catalog No.:BCC6260
CAS No.:1345858-76-5
- 5,7-Di-O-methylquercetin
Catalog No.:BCN3386
CAS No.:13459-07-9
- ML 154
Catalog No.:BCC8022
CAS No.:1345964-89-7
- LY2795050
Catalog No.:BCC1719
CAS No.:1346133-08-1
- Planchol E
Catalog No.:BCN6882
CAS No.:1346137-02-7
- SA 57
Catalog No.:BCC6280
CAS No.:1346169-63-8
- ML 218 hydrochloride
Catalog No.:BCC6207
CAS No.:1346233-68-8
- Stavudine sodium
Catalog No.:BCC4263
CAS No.:134624-73-0
- Eriocitrin
Catalog No.:BCN1208
CAS No.:13463-28-0
A549 cells as a model to study endogenous LPA1 receptor signaling and regulation.[Pubmed:28943105]
Eur J Pharmacol. 2017 Nov 15;815:258-265.
Lysophosphatidic acid (LPA) modulates the function of many organs, including the lung. A549 is a lung carcinoma-derived cell line, frequently used as a model for type II pneumocytes. Here we show that these cells expressed messenger RNA coding for LPA1-3 receptors with the following order of abundance: LPA1 > LPA2 > LPA3 and that LPA was able to increase intracellular calcium, extracellular signal-regulated kinases 1/2 phosphorylation, and cell contraction. These effects were blocked by Ki16425, an antagonist selective for LPA1 and LPA3 receptors, and by the LPA1-selective antagonist, AM095. Activation of protein kinase C inhibited LPA-induced intracellular calcium increase. This action was blocked by protein kinase C inhibitors and enzyme down-regulation. Phorbol myristate acetate and AM095, but not Ki16425, decreased the baseline intracellular calcium concentration. Ki16425 blocked the effect of AM095 but not that of phorbol myristate acetate. The data indicate that LPA1 receptors exhibit constitutive activity and that AM095 behaves as an inverse agonist, whereas Ki16425 appears to be a classic antagonist. Furthermore, the LPA agonist, 1-oleoyl-2-O-methyl-rac-glycerophosphothionate, OMPT, induced a weak increase in intracellular calcium, but was able to induce full ERK 1/2 phosphorylation and cell contraction. These effects were blocked by AM095. These data suggest that OMPT is a biased LPA1 agonist. A549 cells express functional LPA1 receptors and seem to be a suitable model to study their signaling and regulation.
Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma.[Pubmed:21305523]
Arthritis Rheum. 2011 May;63(5):1405-15.
OBJECTIVE: Scleroderma (systemic sclerosis [SSc]), is characterized by progressive multiorgan fibrosis. We recently implicated lysophosphatidic acid (LPA) in the pathogenesis of pulmonary fibrosis. The purpose of the present study was to investigate the roles of LPA and two of its receptors, LPA(1) and LPA(2), in dermal fibrosis in a mouse model of SSc. METHODS: Wild type (WT), and LPA(1)-knockout (KO) and LPA(2)-KO mice were injected subcutaneously with bleomycin or phosphate buffered saline (PBS) once daily for 28 days. Dermal thickness, collagen content, and numbers of cells positive for alpha-smooth muscle actin (alpha-SMA) or phospho-Smad2 were determined in bleomycin-injected and PBS-injected skin. In separate experiments, a novel selective LPA(1) antagonist AM095 or vehicle alone was administered by oral gavage to C57BL/6 mice that were challenged with 28 daily injections of bleomycin or PBS. AM095 or vehicle treatments were initiated concurrently with, or 7 or 14 days after, the initiation of bleomycin and PBS injections and continued to the end of the experiments. Dermal thickness and collagen content were determined in injected skin. RESULTS: The LPA(1) -KO mice were markedly resistant to bleomycin-induced increases in dermal thickness and collagen content, whereas the LPA(2)-KO mice were as susceptible as the WT mice. Bleomycin-induced increases in dermal alpha-SMA+ and phospho-Smad2+ cells were abrogated in LPA(1)-KO mice. Pharmacologic antagonism of LPA(1) with AM095 significantly attenuated bleomycin-induced dermal fibrosis when administered according to either a preventive regimen or two therapeutic regimens. CONCLUSION: These results suggest that LPA/LPA(1) pathway inhibition has the potential to be an effective new therapeutic strategy for SSc, and that LPA(1) is an attractive pharmacologic target in dermal fibrosis.
Intercellular Lipid Mediators and GPCR Drug Discovery.[Pubmed:24404331]
Biomol Ther (Seoul). 2013 Nov;21(6):411-22.
G-protein-coupled receptors (GPCR) are the largest superfamily of receptors responsible for signaling between cells and tissues, and because they play important physiological roles in homeostasis, they are major drug targets. New technologies have been developed for the identification of new ligands, new GPCR functions, and for drug discovery purposes. In particular, intercellular lipid mediators, such as, lysophosphatidic acid and sphingosine 1-phosphate have attracted much attention for drug discovery and this has resulted in the development of fingolimod (FTY-720) and AM095. The discovery of new intercellular lipid mediators and their GPCRs are discussed from the perspective of drug development. Lipid GPCRs for lysophospholipids, including lysophosphatidylserine, lysophosphatidylinositol, lysophosphatidylcholine, free fatty acids, fatty acid derivatives, and other lipid mediators are reviewed.
Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor-selective antagonist.[Pubmed:21159750]
J Pharmacol Exp Ther. 2011 Mar;336(3):693-700.
Lysophosphatidic acid (LPA) is a bioactive phospholipid that signals through a family of at least six G protein-coupled receptors designated LPA(1)(-)(6). LPA type 1 receptor (LPA(1)) exhibits widespread tissue distribution and regulates a variety of physiological and pathological cellular functions. Here, we evaluated the in vitro pharmacology, pharmacokinetic, and pharmacodynamic properties of the LPA(1)-selective antagonist AM095 (sodium, {4'-[3-methyl-4-((R)-1-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}- acetate) and assessed the effects of AM095 in rodent models of lung and kidney fibrosis and dermal wound healing. In vitro, AM095 was a potent LPA(1) receptor antagonist because it inhibited GTPgammaS binding to Chinese hamster ovary (CHO) cell membranes overexpressing recombinant human or mouse LPA(1) with IC(5)(0) values of 0.98 and 0.73 muM, respectively, and exhibited no LPA(1) agonism. In functional assays, AM095 inhibited LPA-driven chemotaxis of CHO cells overexpressing mouse LPA(1) (IC(5)(0)= 778 nM) and human A2058 melanoma cells (IC(5)(0) = 233 nM). In vivo, we demonstrated that AM095: 1) had high oral bioavailability and a moderate half-life and was well tolerated at the doses tested in rats and dogs after oral and intravenous dosing, 2) dose-dependently reduced LPA-stimulated histamine release, 3) attenuated bleomycin-induced increases in collagen, protein, and inflammatory cell infiltration in bronchalveolar lavage fluid, and 4) decreased kidney fibrosis in a mouse unilateral ureteral obstruction model. Despite its antifibrotic activity, AM095 had no effect on normal wound healing after incisional and excisional wounding in rats. These data demonstrate that AM095 is an LPA(1) receptor antagonist with good oral exposure and antifibrotic activity in rodent models.


