AZD3759EGFR inhibitor,oral active CAS# 1626387-80-1 |
- 17 alpha-propionate
Catalog No.:BCC1296
CAS No.:19608-29-8
- Andarine
Catalog No.:BCC1168
CAS No.:401900-40-1
- MDV3100 (Enzalutamide)
Catalog No.:BCC1268
CAS No.:915087-33-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1626387-80-1 | SDF | Download SDF |
| PubChem ID | 78209992 | Appearance | Powder |
| Formula | C22H23ClFN5O3 | M.Wt | 459.90 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (108.72 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
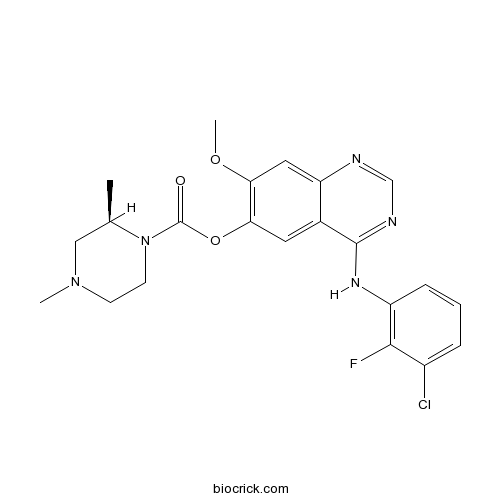
| Chemical Name | [4-(3-chloro-2-fluoroanilino)-7-methoxyquinazolin-6-yl] (2R)-2,4-dimethylpiperazine-1-carboxylate | ||
| SMILES | CC1CN(CCN1C(=O)OC2=C(C=C3C(=C2)C(=NC=N3)NC4=C(C(=CC=C4)Cl)F)OC)C | ||
| Standard InChIKey | MXDSJQHFFDGFDK-CYBMUJFWSA-N | ||
| Standard InChI | InChI=1S/C22H23ClFN5O3/c1-13-11-28(2)7-8-29(13)22(30)32-19-9-14-17(10-18(19)31-3)25-12-26-21(14)27-16-6-4-5-15(23)20(16)24/h4-6,9-10,12-13H,7-8,11H2,1-3H3,(H,25,26,27)/t13-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AZD3759 is a potent, oral active, central nervous system-penetrant, EGFR inhibitor. At Km ATP concentrations, the IC50s are 0.3, 0.2, and 0.2 nM for EGFRwt, EGFRL858R, and EGFRexon 19Del, respectively.In Vitro:At 2 mM of ATP concentrations, the IC50s are 102, 7.6, and 2.4 nM for EGFRwt, EGFRL858R, and EGFRexon 19Del, respectively. AZD3759 also inhibits pEGFR in H838wt, H3255L858R, and PC-9exon 19Del with IC50 of 64.5, 7.2, and 7.4 nM, respectively. In cellular phosphorylation studies, AZD3759 also demonstrates 9-fold inhibition selectivity in EGFR-activating mutant cell lines over EGFR wild-type cell lines (H838 cell line)[1].In Vivo:Following oral dosing in rats at 2 mg/kg, absorption of AZD3759 is rapid with blood Cmax of 0.58 μM achieved at 1.0 h. Subsequently, blood concentrations of AZD3759 decline monoexponentially with a mean elimination half-life of 4.3 h, which is close to the same parameter obtained from intravenous dosing of 4.1 h. The bioavailability following an oral dose in rats is 91%. Blood pharmacokinetic parameters of AZD3759 in male dogs are determined following both a single dose intravenous infusion and oral administration. Following the IV dose in dogs, AZD3759 blood clearance is determined as 14 mL/min per kg, and the volume of distribution is 6.4 L/kg. Its elimination half-life is 6.2 h. Absorption of AZD3759 is rapid with blood Cmax (698 nM) occurring between 0.5 and 1.5 h. The oral bioavailability of AZD3759 is excellent at 90%. AZD3759 demonstrated significant dose-dependent antitumor efficacy (78% tumor growth inhibition at 7.5 mg/kg qd and tumor regression at 15 mg/kg qd, respectively, 4 weeks after treatment) with <20% body weight loss, whereas erlotinib had a limited effect in this model. At the end of the study, brain tissues are collected for histological assessment. Significantly decreased tumor area is observed by AZD3759 treatment at the doses of 7.5 and 15 mg/kg. In addition, modulation of pEGFR is detected by a single dose of AZD3759 at 15 mg/kg 1h after dosing, which confirmed target engagement by AZD3759[1]. References: | |||||

AZD3759 Dilution Calculator

AZD3759 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1744 mL | 10.8719 mL | 21.7439 mL | 43.4877 mL | 54.3596 mL |
| 5 mM | 0.4349 mL | 2.1744 mL | 4.3488 mL | 8.6975 mL | 10.8719 mL |
| 10 mM | 0.2174 mL | 1.0872 mL | 2.1744 mL | 4.3488 mL | 5.436 mL |
| 50 mM | 0.0435 mL | 0.2174 mL | 0.4349 mL | 0.8698 mL | 1.0872 mL |
| 100 mM | 0.0217 mL | 0.1087 mL | 0.2174 mL | 0.4349 mL | 0.5436 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AZD3759 is a potent and oral active inhibitor of EGFR (IC50= 7.0-7.7 nM) with antineoplastic activity.
EGFR (epidermal growth factor receptor) is a cell-surface receptor tyrosine kinase. The receptor activation leads to dimerization and tyrosine autophosphorylation. It induces a cascade of downstream cellular responses such as modification in gene expression, cell proliferation and cytoskeletal rearrangement etc.
AZD3759 shows equally potent inhibitory effect on cell phosphorylation or proliferation in EGFR-activating mutant cell lines (PC-9 and H3255) in the range of 7.0−7.7nM. In cellular phosphorylation assays, AZD3759 also exhibits 9-fold inhibition selectivity in EGFR-activating
mutant cell lines over EGFR wild-type cell lines (H838). [1]
In brain metastasis mouse model, AZD3759 demonstrates prominent antitumor efficacy in a dose dependent manner (∼78% tumor growth inhibition at 7.5 mg/kg qd and tumor regression at 15 mg/kg qd, respectively, 4 weeks after treatment, with <20% body weight loss). In addition, at the doses of 7.5 and 15 mg/k, AZD3759 significantly decreases tumor area in the brain tissue collected from the same mouse model. [1]
Reference:
Zeng Q, Wang J, Cheng Z et al. Discovery and Evaluation of Clinical Candidate AZD3759, a Potent, Oral Active, Central Nervous System-Penetrant, Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. J Med Chem. 2015 Oct 22;58(20):8200-15
- Temsirolimus
Catalog No.:BCC3678
CAS No.:162635-04-3
- Sorokinianin
Catalog No.:BCN6978
CAS No.:162616-73-1
- Eriosemation
Catalog No.:BCN3738
CAS No.:162616-72-0
- Isolupalbigenin
Catalog No.:BCN6835
CAS No.:162616-70-8
- 3-Hydroxy-5,7-dimethoxy-3',4'-methylenedioxyflavan
Catalog No.:BCN1540
CAS No.:162602-04-2
- Broussoflavonol F
Catalog No.:BCN3571
CAS No.:162558-94-3
- Fmoc-Dap(Boc)-OH
Catalog No.:BCC3188
CAS No.:162558-25-0
- CDP 840 hydrochloride
Catalog No.:BCC7814
CAS No.:162542-90-7
- Salirasib
Catalog No.:BCC1918
CAS No.:162520-00-5
- Subelliptenone G
Catalog No.:BCN1720
CAS No.:162473-22-5
- VR23
Catalog No.:BCC6523
CAS No.:1624602-30-7
- Stilbostemin B
Catalog No.:BCN4697
CAS No.:162411-67-8
- AT 56
Catalog No.:BCC6036
CAS No.:162640-98-4
- HQL 79
Catalog No.:BCC7703
CAS No.:162641-16-9
- 6-Deoxyjacareubin
Catalog No.:BCN6573
CAS No.:16265-56-8
- Kaempferol tetraacetate
Catalog No.:BCN1721
CAS No.:16274-11-6
- 3,4-Dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran
Catalog No.:BCN1539
CAS No.:16274-33-2
- 1-Hydroxy-2-prenylnaphthalene
Catalog No.:BCN1722
CAS No.:16274-34-3
- AZD8186
Catalog No.:BCC6470
CAS No.:1627494-13-6
- LDN-214117
Catalog No.:BCC5528
CAS No.:1627503-67-6
- Anemarrhena B
Catalog No.:BCN7592
CAS No.:1627521-95-2
- WAY-100635
Catalog No.:BCC2053
CAS No.:162760-96-5
- Yunnancoronarin A
Catalog No.:BCN1723
CAS No.:162762-93-8
- PFI-2
Catalog No.:BCC5561
CAS No.:1627676-59-8
Discovery and Evaluation of Clinical Candidate AZD3759, a Potent, Oral Active, Central Nervous System-Penetrant, Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor.[Pubmed:26313252]
J Med Chem. 2015 Oct 22;58(20):8200-15.
Recent reports suggest that an increasing number of patients with lung cancer, especially those with activating mutations of the epidermal growth factor receptor (EGFR), also present with brain metastases and leptomeningeal metastases. These patients have poor prognosis as there are no approved drugs for these indications. Available agents have poor efficacy for these patients even at well above their standard dose. Herein, we report the discovery of (4-[(3-chloro-2-fluorophenyl)amino]-7-methoxyquinazolin-6-yl (2R)-2,4-dimethylpiperazine-1-carboxylate 1m (AZD3759), an investigational drug currently in Phase 1 clinical trial, which has excellent central nervous system penetration and which induces profound regression of brain metastases in a mouse model.
AZD3759, a BBB-penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases.[Pubmed:27928026]
Sci Transl Med. 2016 Dec 7;8(368):368ra172.
Non-small-cell lung cancer patients with activating mutations in epidermal growth factor receptor (EGFR) respond to EGFR tyrosine kinase inhibitor (TKI) treatment. Nevertheless, patients often develop central nervous system (CNS) metastases during treatment, even when their extracranial tumors are still under control. In the absence of effective options, much higher doses of EGFR TKIs have been attempted clinically, with the goal of achieving high enough drug concentrations within the CNS. Although limited tumor responses have been observed with this approach, the toxicities outside the CNS have been too high to tolerate. We report the discovery and early clinical development of AZD3759, a selective EGFR inhibitor that can fully penetrate the blood-brain barrier (BBB), with equal free concentrations in the blood, cerebrospinal fluid, and brain tissue. Treatment with AZD3759 causes tumor regression in subcutaneous xenograft, leptomeningeal metastasis (LM), and brain metastasis (BM) lung cancer models and prevents the development of BM in nude mice. An early clinical study in patients with BM and LM treated with AZD3759 confirms its BBB-penetrant properties and antitumor activities. Our data demonstrate the potential of AZD3759 for the treatment of BM and LM and support its further clinical evaluation in larger trials.


