AZD6244 (Selumetinib)MEK inhibitor CAS# 606143-52-6 |
- GDC-0623
Catalog No.:BCC4150
CAS No.:1168091-68-6
- Trametinib DMSO solvate
Catalog No.:BCC2013
CAS No.:1187431-43-1
- Pimasertib (AS-703026)
Catalog No.:BCC2529
CAS No.:1236699-92-5
- SL-327
Catalog No.:BCC1123
CAS No.:305350-87-2
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
- Arctigenin
Catalog No.:BCN6291
CAS No.:7770-78-7
Quality Control & MSDS
Number of papers citing our products

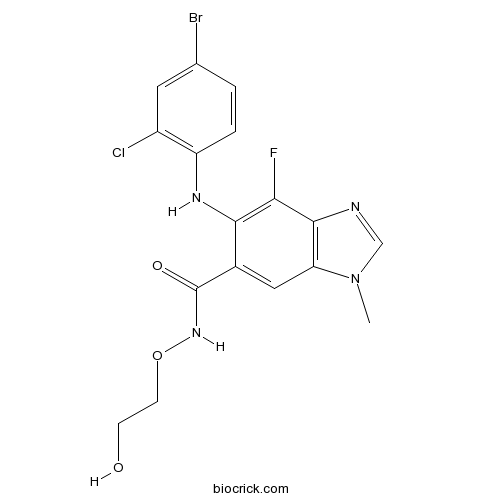
Chemical structure
3D structure
| Cas No. | 606143-52-6 | SDF | Download SDF |
| PubChem ID | 10127622 | Appearance | Powder |
| Formula | C17H15BrClFN4O3 | M.Wt | 457.69 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AZD6244; ARRY-142886 | ||
| Solubility | DMSO : 20.83 mg/mL (45.51 mM; Need ultrasonic) | ||
| Chemical Name | 6-(4-bromo-2-chloroanilino)-7-fluoro-N-(2-hydroxyethoxy)-3-methylbenzimidazole-5-carboxamide | ||
| SMILES | CN1C=NC2=C1C=C(C(=C2F)NC3=C(C=C(C=C3)Br)Cl)C(=O)NOCCO | ||
| Standard InChIKey | CYOHGALHFOKKQC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H15BrClFN4O3/c1-24-8-21-16-13(24)7-10(17(26)23-27-5-4-25)15(14(16)20)22-12-3-2-9(18)6-11(12)19/h2-3,6-8,22,25H,4-5H2,1H3,(H,23,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selumetinib (AZD6244) is a potent, highly selective inhibitor of MEK1 with IC50 of 14 nM. | |||||
| Targets | MEK1 | |||||
| IC50 | 14 nM | |||||
| Cell experiment: [1] | |
| Cell lines | 1205Lu cells (BRAFV600E) |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 3 μM, 24 hours |
| Applications | Inhibition of cell growth by AZD6244 is caused by reversible G1 -phase cell cycle arrest. Adherent 1205Lu cells were treated with DMSO or 3 μM AZD6244 for 24 h or for 24 h and a further 24 h after removal of the drugs. Cells treated with AZD6244 were found to enter into the G1 -phase cell cycle arrest, but to reenter S phase after removal of the drug. |
| Animal experiment: [2] | |
| Animal models | Nude mice implanted with HT-29 human colon carcinoma |
| Dosage form | Oral administration, 10, 25, 50, or 100 mg/kg, twice a day for 21 days |
| Application | AZD6244 is effective in inhibiting tumor growth at all doses tested. The time to the tumor growth end point was 36 days for the two highest dose groups compared with 18 days for the vehicle control group. Tumor growth after 11 days of dosing was inhibited by 55% at the low dose of 10 mg/kg and by 70% at the high dose of 100 mg/kg. Recovery of tumor growth was observed after cessation of AZD6244 administration. Tumor regrowth was significantly delayed in the 100 mg/kg dose group. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Haass N K, Sproesser K, Nguyen T K, et al. The mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor AZD6244 (ARRY-142886) induces growth arrest in melanoma cells and tumor regression when combined with docetaxel. Clinical Cancer Research, 2008, 14(1): 230-239. [2] Yeh T C, Marsh V, Bernat B A, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clinical Cancer Research, 2007, 13(5): 1576-1583. | |

AZD6244 (Selumetinib) Dilution Calculator

AZD6244 (Selumetinib) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1849 mL | 10.9244 mL | 21.8488 mL | 43.6977 mL | 54.6221 mL |
| 5 mM | 0.437 mL | 2.1849 mL | 4.3698 mL | 8.7395 mL | 10.9244 mL |
| 10 mM | 0.2185 mL | 1.0924 mL | 2.1849 mL | 4.3698 mL | 5.4622 mL |
| 50 mM | 0.0437 mL | 0.2185 mL | 0.437 mL | 0.874 mL | 1.0924 mL |
| 100 mM | 0.0218 mL | 0.1092 mL | 0.2185 mL | 0.437 mL | 0.5462 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AZD6244 is a highly potent and selective inhibitor of MEK1/2 with IC50 value of 14.1nM against MEK1 [1].
AZD6244 is a second generation MEK1/2 inhibitor. In the radioactive assay, AZD6244 shows potent inhibition against the purified MEK1without the competition with ATP. Besides, it is a selective inhibitor since it has no obvious inhibition against other tyrosine kinases including MKK6, EGFR, ErbB2 and B-Raf et al. In the cellular assay, AZD6244 inhibits the phosphorylation of ERK1/2 which are the direct substrates of MEK1/2. The IC50 value is 10.3nM. It also inhibits the EGF-induced phosphorylation of ERK1/2 but not ERK5 in A431 cells. Since MEK is important in cell proliferation, the block of MEK1/2 caused by AZD6244 leads to a growth inhibition in the cell lines containing activating B-Raf and Ras mutations with IC50 values ranging from 59 to 473nM. Furthermore, the administration of AZD6244 can significantly inhibit tumor growth both in the HT-29 xenograft model and the BxPC3 pancreatic tumor xenograft model [1].
References:
[1] Yeh TC, Marsh V, Bernat BA, Ballard J, Colwell H, Evans RJ, Parry J, Smith D, Brandhuber BJ, Gross S, Marlow A, Hurley B, Lyssikatos J, Lee PA, Winkler JD, Koch K, Wallace E. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007 Mar 1;13(5):1576-83.
- Momor-cerebroside I
Catalog No.:BCN4120
CAS No.:606125-07-9
- MK-0773
Catalog No.:BCC1754
CAS No.:606101-58-0
- Homopterocarpin
Catalog No.:BCN4615
CAS No.:606-91-7
- Cinnabarinic acid
Catalog No.:BCC7865
CAS No.:606-59-7
- Toyocamycin
Catalog No.:BCC8047
CAS No.:606-58-6
- 2,4'-Dihydroxybenzophenone
Catalog No.:BCN3358
CAS No.:606-12-2
- Pamabrom
Catalog No.:BCC1835
CAS No.:606-04-2
- Tirandamycin B
Catalog No.:BCN1862
CAS No.:60587-14-6
- DCEBIO
Catalog No.:BCC7060
CAS No.:60563-36-2
- P1075
Catalog No.:BCC7027
CAS No.:60559-98-0
- 1-Acetyltagitinin A
Catalog No.:BCN4119
CAS No.:60547-63-9
- Gomisin D
Catalog No.:BCN2268
CAS No.:60546-10-3
- MEK162 (ARRY-162, ARRY-438162)
Catalog No.:BCC1148
CAS No.:606143-89-9
- Bifonazole
Catalog No.:BCC4766
CAS No.:60628-96-8
- Danaidone
Catalog No.:BCN1966
CAS No.:6064-85-3
- Dihydromollugin
Catalog No.:BCN8247
CAS No.:60657-93-4
- 3-n-Butylphthalide
Catalog No.:BCN2381
CAS No.:6066-49-5
- HOSu
Catalog No.:BCC2845
CAS No.:6066-82-6
- Isopicropodophyllone
Catalog No.:BCN8316
CAS No.:60660-50-6
- 3-Hydroxy-1,5-diphenyl-1-pentanone
Catalog No.:BCN3536
CAS No.:60669-64-9
- FC 131
Catalog No.:BCC7917
CAS No.:606968-52-9
- Sesamin
Catalog No.:BCN4123
CAS No.:607-80-7
- Myristicin
Catalog No.:BCN2730
CAS No.:607-91-0
- Physoperuvine
Catalog No.:BCN1402
CAS No.:60723-27-5
Autophagy regulates Selumetinib (AZD6244) induced-apoptosis in colorectal cancer cells.[Pubmed:27448918]
Eur J Med Chem. 2016 Oct 21;122:611-618.
OBJECTIVE: As Selumetinib is a MEK1/2 inhibitor that has gained interest as an anti-tumor agent, the present study was designed to investigate autophagy involvement on Selumetinib-induced apoptosis in colorectal cancer (CRC) cells. METHODS: CRC cells death and cycle studies were assessed by AnnexinV-FITC and PI staining, respectively. Autophagy flux was analysed by Western Blot (LC3II and p62 protein levels) and retroviral infection of SW480 cells for siBecn1 RNA interference experiments. Confocal microscopy was used to determine mCherry-EGFP-LC3 distribution. KEY FINDINGS: The Selumetinib effects were concentration-dependent in SW480 cell line. Whereas 1 muM exerted an arrest in the cell cycle (G1 phase), higher concentrations (10 muM) induced cell death, which was accompanied by autophagy blockage in its last stages. Autophagy induction by Rapamycin (RAPA) increased cell survival, whereas pharmacology autophagy inhibition by Bafilomycin A1 (BAF), Chloroquine (CQ) or 3-Methyladenine (3-MA) increased Selumetinib-induced CRC cells death. CONCLUSIONS: Altogether, these results suggest that autophagy plays a fundamental role in CRC cells response to Selumetinib. In addition, the combination of Selumetinib with autophagy inhibitors may be a useful therapeutic strategy to enhance its activity against colorectal tumours.
A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: a Pediatric Brain Tumor Consortium (PBTC) study.[Pubmed:28339824]
Neuro Oncol. 2017 Aug 1;19(8):1135-1144.
Background: Activation of the mitogen-activated protein kinase pathway is important for growth of pediatric low-grade gliomas (LGGs). The aim of this study was to determine the recommended phase II dose (RP2D) and the dose-limiting toxicities (DLTs) of the MEK inhibitor selumetinib in children with progressive LGG. Methods: Selumetinib was administered orally starting at 33 mg/m2/dose b.i.d., using the modified continual reassessment method. Pharmacokinetic analysis was performed during the first course. BRAF aberrations in tumor tissue were determined by real-time polymerase chain reaction and fluorescence in situ hybridization. Results: Thirty-eight eligible subjects were enrolled. Dose levels 1 and 2 (33 and 43 mg/m2/dose b.i.d.) were excessively toxic. DLTs included grade 3 elevated amylase/lipase (n = 1), headache (n = 1), mucositis (n = 2), and grades 2-3 rash (n = 6). At dose level 0 (25 mg/m2/dose b.i.d, the RP2D), only 3 of 24 subjects experienced DLTs (elevated amylase/lipase, rash, and mucositis). At the R2PD, the median (range) area under the curve (AUC0-infinity) and apparent oral clearance of selumetinib were 3855 ng*h/mL (1780 to 7250 ng x h/mL) and 6.5 L x h-1 x m-2 (3.4 to 14.0 L x h-1 x m-2), respectively. Thirteen of 19 tumors had BRAF abnormalities. Among the 5 (20%) of 25 subjects with sustained partial responses, all at the RP2D, 4 had BRAF aberrations, 1 had insufficient tissue. Subjects received a median of 13 cycles (range: 1-26). Fourteen (37%) completed all protocol treatment (26 cycles [n = 13], 13 cycles [n = 1]) with at least stable disease; 2-year progression-free survival at the RP2D was 69 +/- SE 9.8%. Conclusion: Selumetinib has promising antitumor activity in children with LGG. Rash and mucositis were the most common DLTs.
A phase Ib study of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib in advanced hepatocellular carcinoma (HCC).[Pubmed:27681866]
Ann Oncol. 2016 Dec;27(12):2210-2215.
BACKGROUND: Treatment with sorafenib, although associated with inhibition of tumour growth and angiogenesis in in vivo studies, leads to up-regulation of pERK. The addition of MEK inhibition could potentially abrogate this effect and potentiate anti-tumour activity. This phase I study investigated the maximum tolerated dose (MTD), safety, tolerability, pharmacokinetics (PK) and biomarker correlates of selumetinib combined with sorafenib in patients with advanced hepatocellular carcinoma (HCC). METHODS: Patients with Child-Pugh (CP) score /=6 months in seven patients. The median overall survival was 14.4 months. Selumetinib exposures in combination with sorafenib were comparable to other monotherapy studies. A reduction in permeability-surface area product noted in DCE-MRI with treatment correlated with worse survival outcomes. CONCLUSION: The MTD of selumetinib was 75 mg daily when combined with sorafenib 400 mg twice a day in CP


