AflatremCAS# 70553-75-2 |
Quality Control & MSDS
Number of papers citing our products

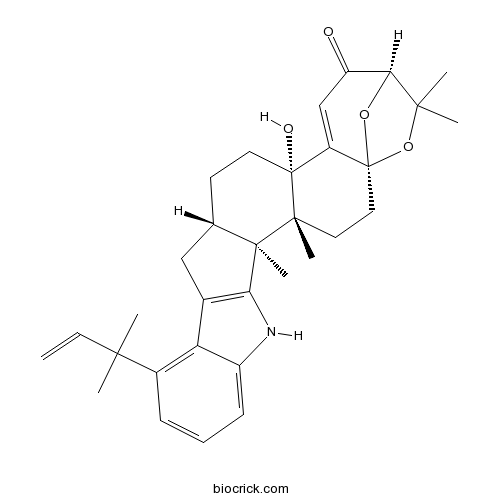
Chemical structure

3D structure
| Cas No. | 70553-75-2 | SDF | Download SDF |
| PubChem ID | 21118293 | Appearance | Powder |
| Formula | C32H39NO4 | M.Wt | 501.66 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1(C2C(=O)C=C3C4(CCC5CC6=C(C5(C4(CCC3(O2)O1)C)C)NC7=CC=CC(=C67)C(C)(C)C=C)O)C | ||
| Standard InChIKey | YVDJBQQJIDPRKP-SLUQHKSNSA-N | ||
| Standard InChI | InChI=1S/C32H39NO4/c1-8-27(2,3)20-10-9-11-21-24(20)19-16-18-12-13-31(35)23-17-22(34)26-28(4,5)37-32(23,36-26)15-14-29(31,6)30(18,7)25(19)33-21/h8-11,17-18,26,33,35H,1,12-16H2,2-7H3/t18-,26-,29+,30+,31+,32-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Aflatrem is a tremorgenic mycotoxin with acute neurotoxic effects, a single low dose of aflatrem is able to induce degeneration of neuronal processes in hippocampal neurotransmitter systems. 2. Aflatrem potentiates the gamma-aminobutyric acid (GABA)-induced chloride current, the potentiating action of aflatrem on the GABAA receptor channel may explain the initial symptoms of intoxication caused by aflatrem in vivo. |
| Targets | GABA Receptor |

Aflatrem Dilution Calculator

Aflatrem Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9934 mL | 9.9669 mL | 19.9338 mL | 39.8676 mL | 49.8345 mL |
| 5 mM | 0.3987 mL | 1.9934 mL | 3.9868 mL | 7.9735 mL | 9.9669 mL |
| 10 mM | 0.1993 mL | 0.9967 mL | 1.9934 mL | 3.9868 mL | 4.9835 mL |
| 50 mM | 0.0399 mL | 0.1993 mL | 0.3987 mL | 0.7974 mL | 0.9967 mL |
| 100 mM | 0.0199 mL | 0.0997 mL | 0.1993 mL | 0.3987 mL | 0.4983 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- NS 3763
Catalog No.:BCC7275
CAS No.:70553-45-6
- α-Conotoxin PnIA
Catalog No.:BCC5978
CAS No.:705300-84-1
- 3,5-Dimethoxybenzylalcohol
Catalog No.:BCN3760
CAS No.:705-76-0
- 2'-Hydroxy-5'-methoxyacetophenone
Catalog No.:BCN4270
CAS No.:705-15-7
- ARP 100
Catalog No.:BCC2370
CAS No.:704888-90-4
- Mitoxantrone HCl
Catalog No.:BCC4924
CAS No.:70476-82-3
- Petasinoside
Catalog No.:BCN1989
CAS No.:70474-34-9
- Petasinine
Catalog No.:BCN1988
CAS No.:70474-33-8
- Schizanthine A
Catalog No.:BCN1936
CAS No.:70474-24-7
- Corymbol
Catalog No.:BCN6617
CAS No.:7047-54-3
- Norfloxacin
Catalog No.:BCC4688
CAS No.:70458-96-7
- Pefloxacin Mesylate
Catalog No.:BCC4821
CAS No.:70458-95-6
- Daurisoline
Catalog No.:BCN2675
CAS No.:70553-76-3
- Herbimycin A
Catalog No.:BCC7132
CAS No.:70563-58-5
- Yunaconitine
Catalog No.:BCN6261
CAS No.:70578-24-4
- Shizukanolide A
Catalog No.:BCN8021
CAS No.:70578-36-8
- Obtusin
Catalog No.:BCC8223
CAS No.:70588-05-5
- Chrysoobtusin
Catalog No.:BCC8309
CAS No.:70588-06-6
- 19alpha-Hydroxyfern-7-ene
Catalog No.:BCN7405
CAS No.:70588-12-4
- 14beta-Benzoyloxy-2-deacetylbaccatin VI
Catalog No.:BCN1373
CAS No.:705973-69-9
- Phlorizin dihydrate
Catalog No.:BCN2584
CAS No.:7061-54-3
- Boc-D-Tyr-OH
Catalog No.:BCC3463
CAS No.:70642-86-3
- Anisotropine Methylbromide; Octatropine Methylbromide
Catalog No.:BCC8120
CAS No.:70642-90-9
- Z-D-Lys-OH
Catalog No.:BCC2761
CAS No.:70671-54-4
Aspergillus flavus aswA, a gene homolog of Aspergillus nidulans oefC, regulates sclerotial development and biosynthesis of sclerotium-associated secondary metabolites.[Pubmed:28442441]
Fungal Genet Biol. 2017 Jul;104:29-37.
Aspergillus flavus aswA (AFLA_085170) is a gene encoding a Zn(II)2Cys6 DNA-binding domain and a transcriptional activation domain, DUF3468. Disruption of aswA yielded strains that made a truncated gene transcript and generated a fungus that produced a greatly increased number of sclerotia. These sclerotia were odd-shaped and non-pigmented (white) and different from oval and pigmented (dark brown to black) mature sclerotia. Transcriptomic analysis of the DeltaaswA strain grown on potato dextrose agar plates and Wickerham agar plates showed that expression of clustering genes involved in the biosynthesis of three sclerotium-associated secondary metabolites was down-regulated. These included gene clusters of asparasone, Aflatrem, and aflavarin. In contrast, those of aflatoxin, cyclopiazonic acid and kojic acid were not affected. Metabolite analyses confirmed that the non-pigmented sclerotia contained aflatoxin and cyclopiazonic acid but not other aforementioned metabolites, three asparasone analogs and dihydroxyaflavinine commonly present in mature sclerotia. Impairment in aswA gene function stalls normal sclerotial development, which in turn prevents biosynthesis and accumulation of sclerotium-specific metabolites.
Aflatrem: a tremorgenic mycotoxin with acute neurotoxic effects.[Pubmed:2867895]
Environ Health Perspect. 1985 Oct;62:459-63.
Tremorgenic mycotoxins induce neurologic symptoms ranging from mental confusion to tremors, seizures and death, and are apparently the only class of mycotoxins with significant central nervous system activity. Tremorgens have been implicated in a number of neurologic diseases of cattle collectively known as staggers syndromes, and pose significant agricultural and health problems for both cattle and humans. Although the effects of tremorgens are thought to result from transient perturbations of amino acid neurotransmitter release mechanisms, there is reason to believe that acute exposures to toxins with such synaptic effects may result in degeneration of neuronal fiber processes. To test this hypothesis, rats were given a single tremorgenic (3 mg/kg, IP) dose of Aflatrem, and kinetics of amino acid neurotransmitter uptake was assessed in isolated hippocampal nerve terminals at 1 day, 1 week, and 2 weeks after injection. Results indicate a decrease in the capacity of the GABA and glutamate uptake systems, which was interpreted as a loss of nerve terminals. The affinity constants suggest a decrease in release of these transmitters as well. In addition to its transient influence on transmitter release, a single low dose of Aflatrem is able to induce degeneration of neuronal processes in hippocampal neurotransmitter systems and therefore represents a long-term health threat.
The tremorigen aflatrem is a positive allosteric modulator of the gamma-aminobutyric acidA receptor channel expressed in Xenopus oocytes.[Pubmed:2538710]
Mol Pharmacol. 1989 Mar;35(3):319-23.
Aflatrem, a mycotoxin from Aspergillus flavus, potentiates the gamma-aminobutyric acid (GABA)-induced chloride current. This positive allosteric regulatory action of Aflatrem was quantitatively studied on the GABAA receptor channel expressed in Xenopus oocytes after injection with chick brain mRNA under voltage-clamp conditions. In this model system, Aflatrem potentiates the current induced by 5 microM GABA in a concentration-dependent manner. Half-maximal potentiation was obtained with 2.4 microM Aflatrem and maximal stimulation of the GABA (5 microM) response was more than 10-fold. The potentiation was not associated with a change of the reversal potential of the GABA-induced current. In the presence of 2 microM Aflatrem, the GABA dose-response curve shifted to lower concentrations, with the Ka decreasing from 28 to 7 microM and the Hill coefficient, n, from 1.5 to 0.8, as measured at a membrane potential of -100 mV. At saturating concentration of GABA (250 microM), Aflatrem (10 microM) was still able to enhance the current by about 21%. Further experiments suggest that the site of action of Aflatrem on the GABAA receptor channel complex is different from that of benzodiazepines, pentobarbital, and picrotoxin. Aflatrem (10 microM) had no significant effect on the coexpressed voltage-dependent sodium and calcium channels and on the kainate channel. The potentiating action of Aflatrem on the GABAA receptor channel may explain the initial symptoms of intoxication caused by Aflatrem in vivo, i.e., diminished activity or immobility of the affected animal.


