Azelastine HClCAS# 79307-93-0 |
- AST-1306 TsOH
Catalog No.:BCC4043
CAS No.:1050500-29-2
- Compound 56
Catalog No.:BCC3615
CAS No.:171745-13-4
- AEE788 (NVP-AEE788)
Catalog No.:BCC2520
CAS No.:497839-62-0
- AST-1306
Catalog No.:BCC3727
CAS No.:897383-62-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 79307-93-0 | SDF | Download SDF |
| PubChem ID | 54360 | Appearance | Powder |
| Formula | C22H25Cl2N3O | M.Wt | 418.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (119.51 mM; Need ultrasonic) H2O : 6.67 mg/mL (15.94 mM; Need ultrasonic) | ||
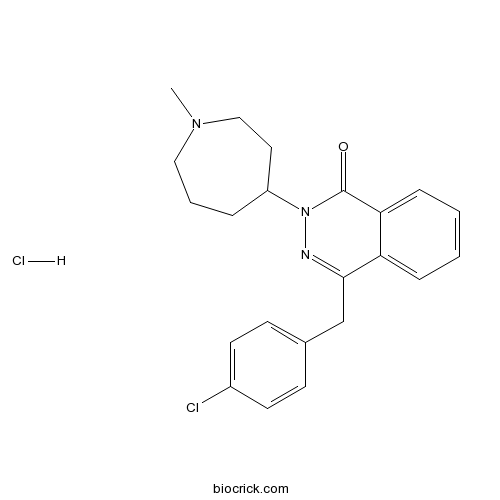
| Chemical Name | 4-[(4-chlorophenyl)methyl]-2-(1-methylazepan-4-yl)phthalazin-1-one;hydrochloride | ||
| SMILES | [H+].[Cl-].CN1CCCC(CC1)N2N=C(Cc3ccc(Cl)cc3)c4ccccc4C2=O | ||
| Standard InChIKey | YEJAJYAHJQIWNU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H24ClN3O.ClH/c1-25-13-4-5-18(12-14-25)26-22(27)20-7-3-2-6-19(20)21(24-26)15-16-8-10-17(23)11-9-16;/h2-3,6-11,18H,4-5,12-15H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Azelastine HCl is a potent, second-generation, selective, histamine antagonist.
Target: Histamine Receptor
Azelastine is a selective H(1)-receptor antagonist that inhibits histamine release and interferes with activation of several other mediators of allergic inflammation. Azelastine can inhibit CHMCs activation and release of IL-6, tryptase, and histamine. On an equimolar basis, azelastine was a more potent inhibitor than olopatadine [1]. Topical azelastine progressively improved itching and conjunctival redness in PAC patients compared to placebo and was at least as effective as levocabastine. Rapid relief is consistent with H(1)-receptor antagonist action, while continued improvement up to 6 weeks may be consistent with mechanisms involving other mediators of allergic inflammation [2]. Azelastine nasal spray was reported to control all rhinitis symptoms, including nasal congestion, regardless of rhinitis diagnosis during the 2-week study period. Patients with seasonal allergic rhinitis and patients with seasonal allergic rhinitis plus nonallergic triggers were identified as patient types most likely to respond to azelastine nasal spray [3]. References: | |||||

Azelastine HCl Dilution Calculator

Azelastine HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3903 mL | 11.9514 mL | 23.9029 mL | 47.8057 mL | 59.7571 mL |
| 5 mM | 0.4781 mL | 2.3903 mL | 4.7806 mL | 9.5611 mL | 11.9514 mL |
| 10 mM | 0.239 mL | 1.1951 mL | 2.3903 mL | 4.7806 mL | 5.9757 mL |
| 50 mM | 0.0478 mL | 0.239 mL | 0.4781 mL | 0.9561 mL | 1.1951 mL |
| 100 mM | 0.0239 mL | 0.1195 mL | 0.239 mL | 0.4781 mL | 0.5976 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Azelastine HCl is a potent, second-generation, selective, histamine antagonist.
- Deoxyandrographolide
Catalog No.:BCN2660
CAS No.:79233-15-1
- SA 47
Catalog No.:BCC6289
CAS No.:792236-07-8
- SB-334867 free base
Catalog No.:BCC5200
CAS No.:792173-99-0
- Cyclosporine
Catalog No.:BCC2559
CAS No.:79217-60-0
- 7-Hydroxyaristolochic acid A
Catalog No.:BCN2659
CAS No.:79185-75-4
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
- Diammonium glycyrrhizinate
Catalog No.:BCN7145
CAS No.:79165-06-3
- A-7 hydrochloride
Catalog No.:BCC6625
CAS No.:79127-24-5
- Hannokinol
Catalog No.:BCN6514
CAS No.:79120-40-4
- Lariciresinol acetate
Catalog No.:BCN4577
CAS No.:79114-77-5
- Polygalasaponin XXXI
Catalog No.:BCN2857
CAS No.:79103-90-5
- (-)-Corlumine
Catalog No.:BCN6632
CAS No.:79082-64-7
- Streptenol E
Catalog No.:BCC8457
CAS No.:
- H-D-Glu-OBzl
Catalog No.:BCC2937
CAS No.:79338-14-0
- Cefixime
Catalog No.:BCC8907
CAS No.:79350-37-1
- AAL Toxin TA2
Catalog No.:BCN1738
CAS No.:79367-51-4
- AAL Toxin TA1
Catalog No.:BCN1733
CAS No.:79367-52-5
- BIBU 1361 dihydrochloride
Catalog No.:BCC7356
CAS No.:793726-84-8
- ent-3Beta-Tigloyloxykaur-16-en-19-oic acid
Catalog No.:BCN1350
CAS No.:79406-09-0
- ent-3beta-Cinnamoyloxykaur-16-en-19-oic acid
Catalog No.:BCN1349
CAS No.:79406-10-3
- 3alpha-Angeloyloxypterokaurene L3
Catalog No.:BCN4576
CAS No.:79406-11-4
- 3alpha-Cinnamoyloxypterokaurene L3
Catalog No.:BCN4575
CAS No.:79406-13-6
- Yadanzioside P
Catalog No.:BCN6711
CAS No.:79439-84-2
- Bruceantinoside A
Catalog No.:BCN7622
CAS No.:79439-85-3
Stability study of the antihistamine drug azelastine HCl along with a kinetic investigation and the identification of new degradation products.[Pubmed:24919676]
Anal Sci. 2014;30(6):691-7.
The first stability-indicating HPLC method was developed and validated for Azelastine HCl (AZL). The separation of AZL from its degradation products was achieved on a C18 column using acetonitrile-0.04 M phosphate buffer of pH 3.5 (32:68, v/v) as a mobile phase with UV-detection at 210 nm and naftazone as an internal standard. The method was rectilinear over the range of 0.2-20.0 mug mL(-1) with a detection limit of 7.05 ng mL(-1). The degradation behavior of AZL was studied under different ICH-recommended stress conditions along with a kinetic investigation; also, degradation products were identified by mass spectrometry. The method was applied for the quality control and stability assessment of AZL in eye drops and nasal spray. The obtained results were favorably compared with those obtained by a comparison method.
[The problem of recurrence of rhino-sinusal polyposis: pilot trial with locally administered azelastine HCL in the prevention of relapses].[Pubmed:8928647]
Acta Otorhinolaryngol Ital. 1995 Apr;15(2):101-6.
Recurrent nasal polyposis is one of the most common unsolved problem in clinical rhinology. In the last few years a great number of histopathological, immunohistochemical and immunological studies on nasal polyps have been carried out by several Authors. Notwithstanding this, the aetiology of these formations still remains unknown. Many data suggest that the presence of polyps is the result of various inflammatory, allergic and pseudo-allergic processes which finally lead to the formation of the oedema constitutive of the polyp itself. In the present report a preliminary trial was carried out in order to evaluate the possibility of preventing recurrences by means of a locally administered anti-H1 receptors drug (Azelastine HCl). In the reported first phase of the study 10 allergic patients with bilateral polyposis were evaluated. Attention was given to the anti-edemigen activity of the drug, as well as to its influence on the local production of Secretory IgA, 7SIgA and albumines. Data are presented and discussed.
Investigation on the efficacy and tolerance of azelastine (HCL) nasal spray versus ebastine tablets in patients with seasonal allergic rhinitis.[Pubmed:8526167]
Allergol Immunopathol (Madr). 1995 Mar-Apr;23(2):51-7.
We compared the efficacy and tolerance of Azelastine nasal spray (0.14 mg in each nostril twice a day) versus Ebastine tablets (10 mg) as a single night dose in a Phase IV open, randomized, parallel-group clinical trial lasting 14 days, conducted with 63 patients diagnosed of seasonal allergic rhinitis. The symptoms assessed before and after the treatment period were: sneezing, nasal pruritus, rhinorrhea, nasal obstruction, conjunctival erythema, eye pruritus, eye watering, photophobia, pharyngeal pruritus and cough. Each symptom was rated by the patients according to a 4-point scale: absent: 0, mild: 1, moderate: 2, and severe: 3. The score required to be included in the study was 8 or above. In addition, the resistance of nasal fossae was assessed, before and after the treatment, by active anterior rhinomanometry, as well as the appearance of adverse events. Both drugs were equally effective both in the control of symptoms and in decreasing the airway resistance and no statistically significant differences were observed in the variables tested in both groups. We concluded that Azelastine nasal spray is a treatment as effective as Ebastine in the relief of symptoms of seasonal allergic rhinitis, with an excellent tolerance and minimum adverse effects.
Validated sensitive spectrofluorimetric method for determination of antihistaminic drug azelastine HCl in pure form and in pharmaceutical dosage forms: application to stability study.[Pubmed:27279096]
Luminescence. 2017 Mar;32(2):177-181.
A highly sensitive, simple and rapid spectrofluorimetric method was developed for the determination of Azelastine HCl (AZL) in either its pure state or pharmaceutical dosage form. The proposed method was based on measuring the native fluorescence of the studied drug in 0.2 M H2 SO4 at lambdaem = 364 nm after excitation at lambdaex = 275 nm. Different experimental parameters were studied and optimized carefully to obtain the highest fluorescence intensity. The proposed method showed a linear dependence of the fluorescence intensity on drug concentration over a concentration range of 10-250 ng/mL, with a limit of detection of 1.52 ng/mL and limit of quantitation of 4.61 ng/mL. Moreover, the method was successfully applied to pharmaceutical preparations, with percent recovery values (+/- SD) of 99.96 (+/- 0.4) and 100.1 (+/- 0.52) for nasal spray and eye drops, respectively. The results were in good agreement with those obtained by the comparison method, as revealed by Student's t-test and the variance ratio F-test. The method was extended to study the stability of AZL under stress conditions, where the drug was exposed to neutral, acidic, alkaline, oxidative and photolytic degradation according to International Conference on Harmonization (ICH) guidelines. Copyright (c) 2016 John Wiley & Sons, Ltd.


