Bacoside A3CAS# 157408-08-7 |
Quality Control & MSDS
Number of papers citing our products

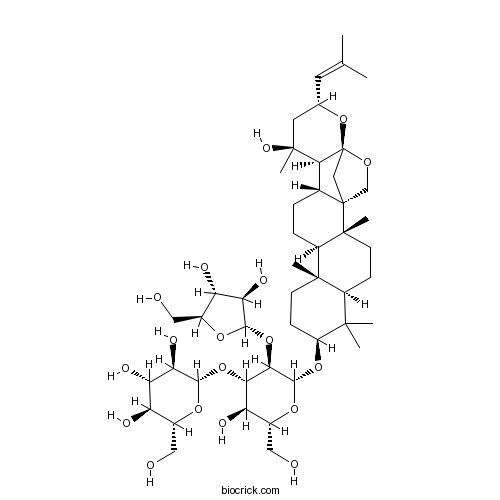
Chemical structure

3D structure
| Cas No. | 157408-08-7 | SDF | Download SDF |
| PubChem ID | 91827005 | Appearance | White powder |
| Formula | C47H76O18 | M.Wt | 929 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | 3-beta-((O-beta-D-glucopyranosyl(1-3)-O-(alpha-L-arabinofuranosyl(1-2))-O-beta-D-glucopyranosyl)oxy)jujubogenin | ||
| Solubility | Soluble in ethanol and methanol; insoluble in chloroform and n-hexane | ||
| Chemical Name | (2S,3R,4S,5S,6R)-2-[(2R,3R,4S,5R,6R)-5-[(2S,3R,4R,5S)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-3-hydroxy-2-(hydroxymethyl)-6-[[(1S,2R,5R,7S,10R,11R,14R,15S,16S,18R,20S)-16-hydroxy-2,6,6,10,16-pentamethyl-18-(2-methylprop-1-enyl)-19,21-dioxahexacyclo[18.2.1.01,14.02,11.05,10.015,20]tricosan-7-yl]oxy]oxan-4-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | CC(=CC1CC(C2C3CCC4C5(CCC(C(C5CCC4(C36CC2(O1)OC6)C)(C)C)OC7C(C(C(C(O7)CO)O)OC8C(C(C(C(O8)CO)O)O)O)OC9C(C(C(O9)CO)O)O)C)(C)O)C | ||
| Standard InChIKey | CDEVGTJBRPBOPH-INTDMYAHSA-N | ||
| Standard InChI | InChI=1S/C47H76O18/c1-21(2)14-22-15-45(7,57)38-23-8-9-28-43(5)12-11-29(42(3,4)27(43)10-13-44(28,6)46(23)19-47(38,65-22)58-20-46)62-41-37(64-39-34(55)31(52)25(17-49)60-39)36(32(53)26(18-50)61-41)63-40-35(56)33(54)30(51)24(16-48)59-40/h14,22-41,48-57H,8-13,15-20H2,1-7H3/t22-,23+,24+,25-,26+,27-,28+,29-,30+,31-,32+,33-,34+,35+,36-,37+,38-,39-,40-,41-,43-,44+,45-,46-,47-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bacoside A3 has antioxidant potential, it shows comparatively higher neuroprotective response analysed as higher cell viability and decreased intracellular ROS. Bacoside A3 shows a newer potential role in the clinical management of opioid withdrawal induced depression. Bacoside A3 inhibited both basal activity as well as verapamil-stimulated ATPase activity, thus its affinity towards P-gp; the interaction of bacosides (A3/A) with Tryptophan hydroxylase (TPH) might up-regulate its activity to elevate the biosynthesis of 5-HT, thereby enhances learning and memory formation. |
| Targets | ROS | P-gp | 5-HT recepter | ATPase |
| In vitro | Comparative evaluation of four triterpenoid glycoside saponins of bacoside A in alleviating sub-cellular oxidative stress of N2a neuroblastoma cells.[Pubmed: 30073654 ]J Pharm Pharmacol. 2018 Nov;70(11):1531-1540.To examine the neuroprotective property of triterpenoid glycoside saponins of Bacopa monnieri (L.) Wettst. bacoside A and its components against H2 O2 -induced oxidative stress on neuronal (N2a) cells.
Molecular docking of bacosides with tryptophan hydroxylase: a model to understand the bacosides mechanism.[Pubmed: 25089244]Nat Prod Bioprospect. 2014 Aug;4(4):251-5.Tryptophan hydroxylase (TPH) catalyses l-tryptophan into 5-hydroxy-l-tryptophan, which is the first and rate-limiting step of serotonin (5-HT) biosynthesis. Earlier, we found that TPH2 up-regulated in the hippocampus of postnatal rats after the oral treatment of Bacopa monniera leaf extract containing the active compound bacosides. However, the knowledge about the interactions between bacosides with TPH is limited. |
| In vivo | Beneficial effects of Bacopa monnieri extract on opioid induced toxicity.[Pubmed: 27441247 ]Heliyon. 2016 Feb 15;2(2):e00068.The present study examined the hepatotoxicity and nephrotoxicity of morphine and illicit street heroin and their amelioration by a standardized methanolic extract of Bacopa monnieri (L.) (mBME) in rats. |
| Kinase Assay | In vitro effects of standardized extract of Bacopa monniera and its five individual active constituents on human P-glycoprotein activity.[Pubmed: 25869246 ]Xenobiotica. 2015;45(8):741-9.1. For centuries Bacopa monniera (BM) has been used as an herbal drug for the treatment of various mental ailments. A chemically standardized alcoholic extract of BM is clinically available over the counter herbal remedy for memory enhancement in children and adults. Consumption of herbal preparations has been reported to alter the function of membrane transporters, especially P-glycoprotein (P-gp), ATP-dependent drug efflux transporter responsible for the development of herb-drug interactions. |
| Animal Research | Inhibitory effect of bacopasides on spontaneous morphine withdrawal induced depression in mice.[Pubmed: 24243728]Phytother Res. 2014 Jun;28(6):937-9.Bacopa monnieri is a perennial herb with a world known image as a nootropic. |

Bacoside A3 Dilution Calculator

Bacoside A3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0764 mL | 5.3821 mL | 10.7643 mL | 21.5285 mL | 26.9107 mL |
| 5 mM | 0.2153 mL | 1.0764 mL | 2.1529 mL | 4.3057 mL | 5.3821 mL |
| 10 mM | 0.1076 mL | 0.5382 mL | 1.0764 mL | 2.1529 mL | 2.6911 mL |
| 50 mM | 0.0215 mL | 0.1076 mL | 0.2153 mL | 0.4306 mL | 0.5382 mL |
| 100 mM | 0.0108 mL | 0.0538 mL | 0.1076 mL | 0.2153 mL | 0.2691 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MAP4
Catalog No.:BCC6758
CAS No.:157381-42-5
- DMP 777
Catalog No.:BCC1534
CAS No.:157341-41-8
- Travoprost
Catalog No.:BCC5189
CAS No.:157283-68-6
- ML-323
Catalog No.:BCC4313
CAS No.:1572414-83-5
- Bosentan Hydrate
Catalog No.:BCC4494
CAS No.:157212-55-0
- 3-Amino-5-phenylpyrazole
Catalog No.:BCC8616
CAS No.:1572-10-7
- Harzianic acid
Catalog No.:BCN1838
CAS No.:157148-06-6
- Noopept
Catalog No.:BCC1804
CAS No.:157115-85-0
- Ajuforrestin A
Catalog No.:BCN8008
CAS No.:157110-18-4
- 3-Amino-4-hydroxybenzoic acid
Catalog No.:BCC8610
CAS No.:1571-72-8
- Lipedoside B-III
Catalog No.:BCC8201
CAS No.:157085-48-8
- SNAP 5089
Catalog No.:BCC7350
CAS No.:157066-77-8
- Taxuspine B
Catalog No.:BCN6938
CAS No.:157414-05-6
- Coronarin D methyl ether
Catalog No.:BCN1705
CAS No.:157528-81-9
- BTS
Catalog No.:BCC5425
CAS No.:1576-37-0
- (S)-SNAP 5114
Catalog No.:BCC7117
CAS No.:157604-55-2
- Boc-Ala-OH
Catalog No.:BCC3047
CAS No.:15761-38-3
- Boc-Pro-OH
Catalog No.:BCC3435
CAS No.:15761-39-4
- NSC 632839 hydrochloride
Catalog No.:BCC2088
CAS No.:157654-67-6
- Prenylpiperitol
Catalog No.:BCN1706
CAS No.:157659-20-6
- 7-Xylosyl-10-deacetylbaccatin III
Catalog No.:BCN7668
CAS No.:157664-03-4
- Perifosine
Catalog No.:BCC3673
CAS No.:157716-52-4
- Gentiournoside D
Catalog No.:BCN7855
CAS No.:157722-21-9
- Gypenoside A
Catalog No.:BCN8459
CAS No.:157752-01-7
HPLC analysis and standardization of Brahmi vati - An Ayurvedic poly-herbal formulation.[Pubmed:24396246]
J Young Pharm. 2013 Sep;5(3):77-82.
OBJECTIVES: The aim of the present study was to standardize Brahmi vati (BV) by simultaneous quantitative estimation of Bacoside A3 and Piperine adopting HPLC-UV method. BV very important Ayurvedic polyherbo formulation used to treat epilepsy and mental disorders containing thirty eight ingredients including Bacopa monnieri L. and Piper longum L. MATERIALS AND METHODS: An HPLC-UV method was developed for the standardization of BV in light of simultaneous quantitative estimation of Bacoside A3 and Piperine, the major constituents of B. monnieri L. and P. longum L. respectively. The developed method was validated on parameters including linearity, precision, accuracy and robustness. RESULTS: The HPLC analysis showed significant increase in amount of Bacoside A3 and Piperine in the in-house sample of BV when compared with all three different marketed samples of the same. Results showed variations in the amount of Bacoside A3 and Piperine in different samples which indicate non-uniformity in their quality which will lead to difference in their therapeutic effects. CONCLUSION: The outcome of the present investigation underlines the importance of standardization of Ayurvedic formulations. The developed method may be further used to standardize other samples of BV or other formulations containing Bacoside A3 and Piperine.
Inhibitory effect of bacopasides on spontaneous morphine withdrawal induced depression in mice.[Pubmed:24243728]
Phytother Res. 2014 Jun;28(6):937-9.
Bacopa monnieri is a perennial herb with a world known image as a nootropic. We investigated the effect of Bacopa monnieri methanolic extract (Mt Ext BM) 10, 20, and 30 mg/kg body weight (b.w) on acquisition and expression of morphine withdrawal induced depression in mice. Locally available Bacopa monnieri (BM) was screened for contents of Bacoside A3, Bacopasaponin C, and Bacopaside II using HPLC with UV. Morphine dependence was induced in mice using twice daily escalating chronic morphine treatments (20-65 mg/kg b.w) for eight consecutive days. Morphine withdrawal induced depression was assayed in animals using forced swimming test (FST), three days after last morphine injection. The HPLC analysis revealed that Mt-ext BM contained Bacoside A3 as major component, i.e. 4 microg in each mg of extract. The chronic treatment with Met Ext BM 10, 20, and 30 mg/kg b.w. dosing significantly inhibited opioid withdrawal induced depression in mice. These findings imply a newer potential role of Bacopa monnieri in the clinical management of opioid withdrawal induced depression which can be attributed to Bacoside A3.


