(-)-Bicuculline methochlorideWater-soluble GABAA antagonist CAS# 53552-05-9 |
- RU 58841
Catalog No.:BCC1911
CAS No.:154992-24-2
- 17 alpha-propionate
Catalog No.:BCC1296
CAS No.:19608-29-8
- Spironolactone
Catalog No.:BCC4366
CAS No.:52-01-7
- MDV3100 (Enzalutamide)
Catalog No.:BCC1268
CAS No.:915087-33-1
- ARN-509
Catalog No.:BCC3724
CAS No.:956104-40-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 53552-05-9 | SDF | Download SDF |
| PubChem ID | 10047593 | Appearance | Powder |
| Formula | C21H20ClNO6 | M.Wt | 417.8 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
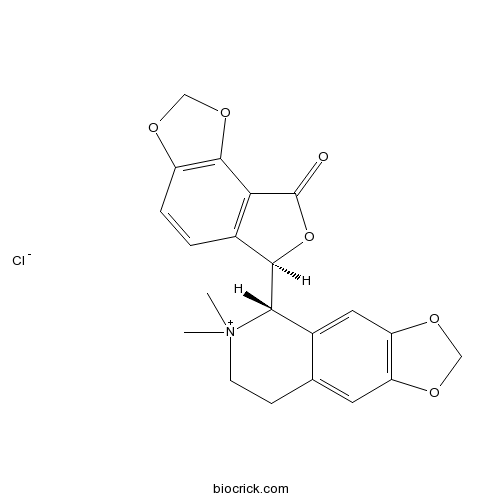
| Chemical Name | (6R)-6-[(5S)-6,6-dimethyl-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]isoquinolin-6-ium-5-yl]-6H-furo[3,4-g][1,3]benzodioxol-8-one;chloride | ||
| SMILES | C[N+]1(CCC2=CC3=C(C=C2C1C4C5=C(C6=C(C=C5)OCO6)C(=O)O4)OCO3)C.[Cl-] | ||
| Standard InChIKey | RLJKFAMYSYWMND-GRTNUQQKSA-M | ||
| Standard InChI | InChI=1S/C21H20NO6.ClH/c1-22(2)6-5-11-7-15-16(26-9-25-15)8-13(11)18(22)19-12-3-4-14-20(27-10-24-14)17(12)21(23)28-19;/h3-4,7-8,18-19H,5-6,9-10H2,1-2H3;1H/q+1;/p-1/t18-,19+;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (-)-Bicuculline methochloride is a GABAA-receptor antagonist. 2. Exposure to 100 microM (-)-Bicuculline methochloride for 48 hr can result in prominent CA1 cell death. |
| Targets | GABA Receptor |

(-)-Bicuculline methochloride Dilution Calculator

(-)-Bicuculline methochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3935 mL | 11.9674 mL | 23.9349 mL | 47.8698 mL | 59.8372 mL |
| 5 mM | 0.4787 mL | 2.3935 mL | 4.787 mL | 9.574 mL | 11.9674 mL |
| 10 mM | 0.2393 mL | 1.1967 mL | 2.3935 mL | 4.787 mL | 5.9837 mL |
| 50 mM | 0.0479 mL | 0.2393 mL | 0.4787 mL | 0.9574 mL | 1.1967 mL |
| 100 mM | 0.0239 mL | 0.1197 mL | 0.2393 mL | 0.4787 mL | 0.5984 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Diaveridine
Catalog No.:BCC8936
CAS No.:5355-16-8
- Benzoyl-L-histidine
Catalog No.:BCC8866
CAS No.:5354-94-9
- Apigenin 7-O-methylglucuronide
Catalog No.:BCN5708
CAS No.:53538-13-9
- Luteolin-3-O-beta-D-glucuronide
Catalog No.:BCN3826
CAS No.:53527-42-7
- Durantoside I
Catalog No.:BCN4621
CAS No.:53526-67-3
- Durantoside II
Catalog No.:BCN4622
CAS No.:53526-66-2
- 7-Amino-4-(Trifluoromethyl)Coumarin
Catalog No.:BCC9208
CAS No.:53518-15-3
- 4,9,9'-Trihydroxy-3,3'-dimethoxy-8,4'-oxyneolignan
Catalog No.:BCN1425
CAS No.:53505-68-3
- PIT 1
Catalog No.:BCC7873
CAS No.:53501-41-0
- Marein
Catalog No.:BCN6477
CAS No.:535-96-6
- Trigonelline
Catalog No.:BCN2371
CAS No.:535-83-1
- Pipecolinic acid
Catalog No.:BCN8150
CAS No.:535-75-1
- Beta-Amyrenonol acetate
Catalog No.:BCN4620
CAS No.:5356-56-9
- 5-Galloylquinic acid
Catalog No.:BCN3060
CAS No.:53584-43-3
- H-Tyr-OBzl.TosOH
Catalog No.:BCC3124
CAS No.:53587-11-4
- Secolongifolenediol
Catalog No.:BCN6989
CAS No.:53587-37-4
- 5'-Iodoresiniferatoxin
Catalog No.:BCC7031
CAS No.:535974-91-5
- Ethionamide
Catalog No.:BCC3778
CAS No.:536-33-4
- Perillyl alcohol
Catalog No.:BCN3876
CAS No.:536-59-4
- 1-Hydroxyrutaecarpine
Catalog No.:BCN5709
CAS No.:53600-24-1
- GMQ hydrochloride
Catalog No.:BCC6351
CAS No.:5361-15-9
- Teucvin
Catalog No.:BCN8375
CAS No.:53625-15-3
- Vindesine
Catalog No.:BCN2607
CAS No.:53643-48-4
- Dehydrobruceantin
Catalog No.:BCN7617
CAS No.:53662-98-9
Effect of low Mg2+ and bicuculline on cell survival in hippocampal slice cultures.[Pubmed:20942591]
Int J Neurosci. 2010 Dec;120(12):752-9.
A reliable model system of epileptiform insult would facilitate investigation into the underlying biological mechanisms. Epileptiform insult was induced in hippocampal slice cultures by lowering extracellular Mg(2+), (+)-bicuculline, or (-)-Bicuculline methochloride, a stable salt form of bicuculline (both forms block GABA(A) receptors). Cell death was assessed by propidium iodide uptake. Low Mg(2+) or (+)-bicuculline did not produce cell death regardless of dose or incubation period. Exposure to 100 microM (-)-Bicuculline methochloride for 48 hr resulted in prominent CA1 cell death. These findings demonstrate that not all pro-epileptic drugs/ion changes used routinely for electrophysiological recording of seizure activity lead to cell death in hippocampal slice cultures and that treatment with bicuculline methochloride can be used as a reliable model for epileptiform insult.
Evidence for a non-GABAergic action of quaternary salts of bicuculline on dopaminergic neurones.[Pubmed:9517436]
Neuropharmacology. 1997 Nov-Dec;36(11-12):1653-7.
Intracellular recordings were made from neurones, presumed to be dopaminergic, in the rat midbrain slice preparation. Bicuculline methiodide (BMI) and methochloride (BMC) reversibly blocked the slow, apamin-sensitive component of the afterhyperpolarization in these cells. The IC50 for this effect was about 26 microM. In comparison, BMC antagonized the input resistance decrease evoked by muscimol (3 microM) with an IC50 of 7 microM. The base of bicuculline was less potent in blocking the slow afterhyperpolarization. SR95531 (2-[carboxy-3'-propyl]-3-amino-6-paramethoxy-phenyl-pyridaziniu m bromide), another competitive GABA(A) antagonist, and picrotoxin, a non-competitive GABA(A) antagonist, also blocked the action of muscimol (IC50s: 2 and 12 microM respectively), but had no effect on the afterhyperpolarization at a concentration of up to 100 microM. Moreover, 100 microM SR95531 did not affect the blockade of the afterhyperpolarization by BMC. This blockade persisted in the presence of tetrodotoxin and was apparently not due to a reduction of calcium entry, suggesting that it involved a direct action on the channels which mediate this afterhyperpolarization. These results strongly suggest that quaternary salts of bicuculline act on more than one target in the central nervous system. Thus, the involvement of GABA(A) receptors in a given effect cannot be proven solely on the basis of its blockade by these agents.
Quantitative evaluation of the potencies of GABA-receptor agonists and antagonists using the rat hippocampal slice preparation.[Pubmed:3011168]
Br J Pharmacol. 1986 Apr;87(4):677-84.
CA1 population spikes recorded in the rat hippocampal slice were used to assess quantitatively the potencies of GABA-receptor agonists and antagonists on mammalian CNS neurones. Apart from GABA itself, GABA A-receptor agonists inhibited the CA1 population spikes with potencies that correlated closely (r = 0.96) with their ability to displace [3H]-GABA from GABAA-binding sites. The low potency of GABA in this preparation was attributed to the action of uptake processes as the GABA uptake inhibitor, cis-4-hydroxynipecotic acid (2 X 10(-4) M), produced an approximate 6 fold increase in the potency of GABA whilst having no effect on the potency of 4,5,6,7-tetrahydroisoxazolo [5,4-c] pyridin-3-ol (THIP), a GABAA-receptor agonist which is not a substrate for the GABA uptake system. The inhibitory effects of the selective GABAA-receptor agonists isoguvacine and muscimol were antagonized by bicuculline methochloride, which shifted the dose-response curves to the right in a parallel manner. The Schild plots for bicuculline methochloride against isoguvacine and muscimol had slopes of 1 and gave pA2 values of 6.24 and 6.10, respectively. Picrotoxin also antagonized the inhibitory effects of isoguvacine and produced parallel shifts to the right of the dose-response curve. However, the Schild plot for picrotoxin had a slope significantly less than unity (0.82) and gave a pA2 value of 6.89. The novel GABAA-receptor antagonist, pitrazepin, antagonized the inhibitory effects of isoguvacine in an apparently competitive manner. The Schild plot had a slope of 1 and gave a pA2 of 6.69. 6 The inhibitory effects of baclofen, GABA and kojic amine were not antagonized by GABAAreceptor antagonists and were presumed to be mediated by actions at GABA5-receptors. 7 The inhibitory effects of THIP and isoguvacine were antagonized with the same potency by bicuculline methobromide. These results do not support the suggestion that THIP acts preferentially at a 'synaptic' bicuculline-sensitive, GABA receptor. 8 It is concluded that the CAI population spike in the rat hippocampal slice is a useful test system for the quantitative analysis of both GABAA- and GABAB-receptor agonists and antagonists.


