CI 966 hydrochlorideSelective inhibitor of GAT-1 CAS# 110283-66-4 |
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- H-1152
Catalog No.:BCC1615
CAS No.:451462-58-1
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 110283-66-4 | SDF | Download SDF |
| PubChem ID | 198692 | Appearance | Powder |
| Formula | C23H22ClF6NO3 | M.Wt | 509.87 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in ethanol and to 100 mM in DMSO | ||
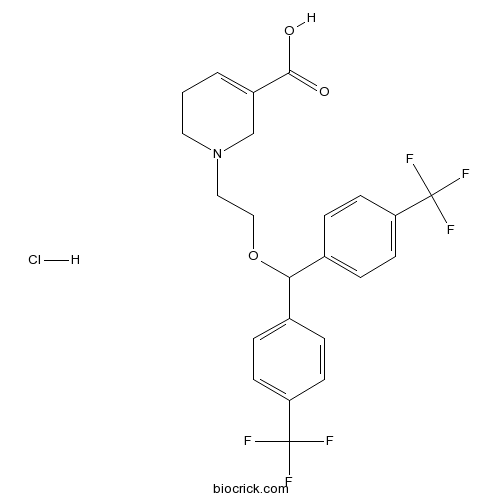
| Chemical Name | 1-[2-[bis[4-(trifluoromethyl)phenyl]methoxy]ethyl]-3,6-dihydro-2H-pyridine-5-carboxylic acid;hydrochloride | ||
| SMILES | C1CN(CC(=C1)C(=O)O)CCOC(C2=CC=C(C=C2)C(F)(F)F)C3=CC=C(C=C3)C(F)(F)F.Cl | ||
| Standard InChIKey | NUQWSOWKRTZJTO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H21F6NO3.ClH/c24-22(25,26)18-7-3-15(4-8-18)20(16-5-9-19(10-6-16)23(27,28)29)33-13-12-30-11-1-2-17(14-30)21(31)32;/h2-10,20H,1,11-14H2,(H,31,32);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of the GABA transporter GAT-1 (IC50 values are 0.26 and 1.2 μM at cloned human and rat GAT-1 respectively). Displays over 200-fold selectivity over GAT-2 and GAT-3. Centrally active upon systemic administration in vivo. Anticonvulsive and neuroprotective. |

CI 966 hydrochloride Dilution Calculator

CI 966 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9613 mL | 9.8064 mL | 19.6128 mL | 39.2257 mL | 49.0321 mL |
| 5 mM | 0.3923 mL | 1.9613 mL | 3.9226 mL | 7.8451 mL | 9.8064 mL |
| 10 mM | 0.1961 mL | 0.9806 mL | 1.9613 mL | 3.9226 mL | 4.9032 mL |
| 50 mM | 0.0392 mL | 0.1961 mL | 0.3923 mL | 0.7845 mL | 0.9806 mL |
| 100 mM | 0.0196 mL | 0.0981 mL | 0.1961 mL | 0.3923 mL | 0.4903 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bacoside A
Catalog No.:BCC8127
CAS No.:11028-00-5
- Agnuside
Catalog No.:BCN5990
CAS No.:11027-63-7
- Amrubicin
Catalog No.:BCC3640
CAS No.:110267-81-7
- Ganoderenic acid E
Catalog No.:BCN8241
CAS No.:110241-23-1
- Ganoderic acid N
Catalog No.:BCN2438
CAS No.:110241-19-5
- (-)-beta-Peltatin-5-O-beta-D-glucopyranoside
Catalog No.:BCN3607
CAS No.:11024-59-2
- Digitonin
Catalog No.:BCN3734
CAS No.:11024-24-1
- Nothofagin
Catalog No.:BCN3787
CAS No.:11023-94-2
- Temocapril HCl
Catalog No.:BCC5016
CAS No.:110221-44-8
- Ginsenoside Rc
Catalog No.:BCN1072
CAS No.:11021-14-0
- Ginsenoside Rb2
Catalog No.:BCN1064
CAS No.:11021-13-9
- Cochliophilin A
Catalog No.:BCC8154
CAS No.:110204-45-0
- Santalol
Catalog No.:BCN8352
CAS No.:11031-45-1
- α-Bungarotoxin
Catalog No.:BCC7264
CAS No.:11032-79-4
- FD-838
Catalog No.:BCN6396
CAS No.:110341-78-1
- CGS 19755
Catalog No.:BCC6986
CAS No.:110347-85-8
- Bavisant dihydrochloride hydrate
Catalog No.:BCC1404
CAS No.:1103522-80-0
- S 32826
Catalog No.:BCC7678
CAS No.:1103672-43-0
- Ch 55
Catalog No.:BCC7241
CAS No.:110368-33-7
- 3-O-Methyltagitinin F
Catalog No.:BCN5991
CAS No.:110382-37-1
- 2,3-Dihydroheveaflavone
Catalog No.:BCN4019
CAS No.:110382-42-8
- Meclizine hydrochloride
Catalog No.:BCC9017
CAS No.:1104-22-9
- Higenamine HCl
Catalog No.:BCN2831
CAS No.:11041-94-4
- Gelsemiol
Catalog No.:BCN5992
CAS No.:110414-77-2
Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1.[Pubmed:7851497]
Eur J Pharmacol. 1994 Oct 14;269(2):219-24.
gamma-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the mammalian brain. The synaptic action of GABA is terminated by rapid uptake into presynaptic terminals and surrounding glial cells. Molecular cloning has revealed the existence of four distinct GABA transporters termed GAT-1, GAT-2, GAT-3, and BGT-1. Pharmacological inhibition of transport provides a mechanism for increasing GABA-ergic transmission, which may be useful in the treatment of various neuropsychiatric disorders. Recently, a number of lipophilic GABA transport inhibitors have been designed and synthesized, which are capable of crossing the blood brain barrier, and which display anticonvulsive activity. We have now determined the potency of four of these compounds, SK&F 89976-A (N-(4,4-diphenyl-3-butenyl)-3-piperidinecarboxylic acid), tiagabine ((R)-1-[4,4-bis(3-methyl-2-thienyl)-3-butenyl]-3- piperidencarboxylic acid), CI-966 ([1-[2-[bis 4-(trifluoromethyl)phenyl]methoxy]ethyl]-1,2,5,6-tetrahydro-3- pyridinecarboxylic acid), and NNC-711 (1-(2-(((diphenylmethylene)amino)oxy)ethyl)-1,2,4,6-tetrahydro-3- pyridinecarboxylic acid hydrochloride), at each of the four cloned GABA transporters, and find them to be highly selective for GAT-1. These data suggest that the anticonvulsant activity of these compounds is mediated via inhibition of uptake by GAT-1.
Pharmacokinetics, mass balance, and induction potential of a novel GABA uptake inhibitor, CI-966 HCl, in laboratory animals.[Pubmed:8272405]
Pharm Res. 1993 Oct;10(10):1442-5.
CI-966 exhibits anticonvulsant properties in various animal models. The drug acts by inhibiting synaptic uptake of gamma-aminobutyric acid (GABA). Oral absorption of CI-966 in dogs given 1.39 mg/kg is rapid with a tmax of 0.7 hr. In rats given 5 mg/kg oral, a mean tmax of 4.0 hr was observed. Following iv administration of the same respective doses, elimination t1/2 in dogs and rats averaged 1.2 and 4.5 hr. Absolute oral bioavailability of CI-966 was 100% in both species. Following oral dosing of [14C]CI-966 HCl to dogs, fecal, and urinary excretion accounted for 89% and 2.3% of the 14C dose, respectively. In bile-duct cannulated rats, biliary excretion is the major elimination pathway of radioactivity (75%). Urinary and fecal excretion accounted for 4.1 and 12%, respectively. CI-966 does not induce or inhibit mouse hepatic mixed function oxidases, as determined by hexobarbital sleeping time.
Systemic CI-966, a new gamma-aminobutyric acid uptake blocker, enhances gamma-aminobutyric acid action in CA1 pyramidal layer in situ.[Pubmed:2276082]
Can J Physiol Pharmacol. 1990 Sep;68(9):1194-9.
A new potent, blood-brain barrier permeable gamma-aminobutyric acid (GABA) uptake blocker, 1-[2-[bis[4-(trifluoromethyl)-phenyl]methoxy]ethyl]-1,2,5,6- tetrahydro-3-pyridinecarboxylic acid (CI-966) was administered systemically by i.p. injection (5 mg/kg) in Sprague-Dawley rats under urethane anaesthesia. Twenty to thirty minutes after injection there was a highly variable, but overall significant, enhancement of the inhibition of hippocampal population spikes by GABA applied by microiontophoresis in the CA1 region. Like the effect of nipecotic acid (applied locally by iontophoresis), the potentiation by CI-966 was clearest when GABA was applied in or near the stratum pyramidale where its action normally is weakest and shows the most pronounced fading. This change in GABA potency is most simply explained by a reduction in GABA uptake.


