ChetominA4505 inhibitor CAS# 1403-36-7 |
- BAY 87-2243
Catalog No.:BCC4131
CAS No.:1227158-85-1
- FG-4592 (ASP1517)
Catalog No.:BCC2227
CAS No.:808118-40-3
- DMOG
Catalog No.:BCC2433
CAS No.:89464-63-1
- KC7F2
Catalog No.:BCC2434
CAS No.:927822-86-4
- IOX2(Glycine)
Catalog No.:BCC2229
CAS No.:931398-72-0
Quality Control & MSDS
Number of papers citing our products

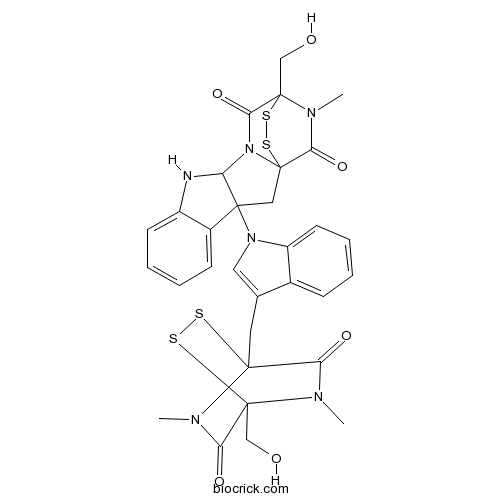
Chemical structure
3D structure
| Cas No. | 1403-36-7 | SDF | Download SDF |
| PubChem ID | 2693 | Appearance | Powder |
| Formula | C31H30N6O6S4 | M.Wt | 710.87 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Limited solubility | ||
| SMILES | CN1C(=O)C2(N(C(=O)C1(SS2)CC3=CN(C4=CC=CC=C43)C56CC78C(=O)N(C(C(=O)N7C5NC9=CC=CC=C69)(SS8)CO)C)C)CO | ||
| Standard InChIKey | ZRZWBWPDBOVIGQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C31H30N6O6S4/c1-33-25(42)30(15-38)34(2)23(40)28(33,44-46-30)12-17-13-36(21-11-7-4-8-18(17)21)27-14-29-24(41)35(3)31(16-39,47-45-29)26(43)37(29)22(27)32-20-10-6-5-9-19(20)27/h4-11,13,22,32,38-39H,12,14-16H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Targets the CH1 domain of CBP/p300; inhibits interaction of HIF-1α, HIF-2α and STAT2 with CBP/p300. Attenuates hypoxia-induced gene expression in vitro and in vivo; radiosensitizes human HT 1080 fibrosarcoma cells in vitro. | |||||

Chetomin Dilution Calculator

Chetomin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4067 mL | 7.0336 mL | 14.0673 mL | 28.1345 mL | 35.1682 mL |
| 5 mM | 0.2813 mL | 1.4067 mL | 2.8135 mL | 5.6269 mL | 7.0336 mL |
| 10 mM | 0.1407 mL | 0.7034 mL | 1.4067 mL | 2.8135 mL | 3.5168 mL |
| 50 mM | 0.0281 mL | 0.1407 mL | 0.2813 mL | 0.5627 mL | 0.7034 mL |
| 100 mM | 0.0141 mL | 0.0703 mL | 0.1407 mL | 0.2813 mL | 0.3517 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Chetomin, an antibiotic metabolite of chaetomium cochliodes, is an inhibitor of HIF-1 by weaken transcription of HIF-1 and recently is raised interests as a cancer chemotherapeutic agent.[1]
HIF-1 (hypoxia-inducible factors 1) is transcription factor which respond to changes of cellular environment oxygen. [2] HIF-1 complex belongs to the PAS subfamily of the basic helix-loop-helix family of transcription factors and is composed of an alpha subunit and a beta subunit which formed a heterodimer. [3] HIF-1 can upregulate several genes such as glycolysis enzymes, vascular endothelial growth factor to promote survival rate of cells in hopoxia conditions through binding to HIF-responsive elements in the promoters. NF- Kappa B was shown a direct modulator of HIF-1alpha in normal condition but in low-oxygen conditions HIF-1alpha still stable with an unknown mechanism. [4]
Chetomin selectively inhibited HIF-1 activities through disruption of the interaction of HIF-1with its transcriptional coactivator p300. Early-passage human fibrosarcoma HT1080 cells stably transfected EGFP plasmid with a hypoxia-responsive 5HRE-hCMVmp promoter were pretreated with chetomin showed dose and incubation-time dependent EGFP fluorescence signal suppression. And pretreatment of 150nM chetomin for 4h showed maximum suppressive effects, indicated the inhibition of HIF-dependent transcription. Also, 150nM chetomin decreased expression of VEGF and CA9 under hapoxic conditions in HT1010 cells. Chetomin increased cell survival rate under hypoxia compared mormoxia conditions, indicated that chetomin can enhanced radiation treatment efficacy under severely hypoxic conditions.[5]
References:
[1] Timothy R. Welch and Robert M. Williams. Studies on the Biosynthesis of Chetomin: Enantiospecific Synthesis of a Putative, Late-Stage Biosynthetic Intermediate. Tetrahedron (2013) 69(2): 770–773
[2] Smith TG, Robbins PA, Ratcliffe PJ. The human side of hypoxia-inducible factor. Br. J. Haematol. (2008)141(3): 325–34
[3] Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J. Biol. Chem. (1996) 271(30):17771-17778
[4] Van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1 alpha by NF-kappa B. Biochem. J. (2008) 412(3): 477-484
[5] Adrian Staab, Jürgen Loeffler, Harun M Said, Désirée Diehlmann, Astrid Katzer, Melanie Beyer, Markus Fleischer, Franz Schwab, Kurt Baier, Hermann Einsele, Michael Flentje and Dirk Vordermark. Effects of HIF-1 inhibition by chetomin on hypoxia-related transcription and radiosensitivity in HT 1080 human fibrosarcoma Cells. BMC Cancer (2007) 7 :213
- NLG919
Catalog No.:BCC2325
CAS No.:1402836-58-1
- N-Methyllindcarpine
Catalog No.:BCN6218
CAS No.:14028-97-8
- Amoxapine
Catalog No.:BCC4624
CAS No.:14028-44-5
- Boc-Lys(2-Cl-Z)-ol
Catalog No.:BCC2581
CAS No.:14028-05-8
- TUG-770
Catalog No.:BCC2018
CAS No.:1402601-82-4
- Eupalinolide I
Catalog No.:BCN7367
CAS No.:1402067-84-8
- Psiguadial D
Catalog No.:BCN7086
CAS No.:1402066-95-8
- GSK2879552
Catalog No.:BCC6422
CAS No.:1401966-69-5
- Vc-MMAD
Catalog No.:BCC2032
CAS No.:1401963-17-4
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- (S)-(-)-5-Iodowillardiine
Catalog No.:BCC6597
CAS No.:140187-25-3
- (S)-(-)-5-Fluorowillardiine
Catalog No.:BCC6596
CAS No.:140187-23-1
- Squalene-2,3-diol
Catalog No.:BCN6220
CAS No.:14031-37-9
- EPZ-6438
Catalog No.:BCC3634
CAS No.:1403254-99-8
- Macranthoidin A
Catalog No.:BCN2808
CAS No.:140360-29-8
- Heteroclitin D
Catalog No.:BCN8166
CAS No.:140369-76-2
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
- Nexturastat A
Catalog No.:BCC5345
CAS No.:1403783-31-2
- 6'-O-Cinnamoyl-8-epikingisidic acid
Catalog No.:BCN7059
CAS No.:1403984-03-1
- Vancomycin hydrochloride
Catalog No.:BCC4232
CAS No.:1404-93-9
- RSVA 405
Catalog No.:BCC8016
CAS No.:140405-36-3
- CCT244747
Catalog No.:BCC6423
CAS No.:1404095-34-6
- ON 146040
Catalog No.:BCC8058
CAS No.:1404231-34-0
- 7-Methoxycoumarin-4-acetyl-P-L-G-L-β-(2,4-dinitrophenylamino)A-R amide
Catalog No.:BCC1086
CAS No.:140430-53-1
Chetomin, targeting HIF-1alpha/p300 complex, exhibits antitumour activity in multiple myeloma.[Pubmed:26867162]
Br J Cancer. 2016 Mar 1;114(5):519-23.
BACKGROUND: Multiple myeloma (MM) is an incurable clonal plasma cell malignancy. The constitutive expression of HIF-1alpha in MM suggests that inhibition of HIF-1alpha-mediated transcription represents an interesting target in MM. METHODS: As p300 is a crucial co-activator of hypoxia-inducible transcription, disrupting the complex HIF-1alpha/p300 to target HIF activity appears to be an attractive strategy. RESULTS: We reported that Chetomin, an inhibitor of HIF-1alpha/p300 interaction, exhibits antitumour activity in human myeloma cell lines and primary MM cells from patients. CONCLUSIONS: Our data suggest that Chetomin may be of clinical value in MM and especially for patients characterised by a high EP300/HIF-1alpha expression and a poor prognosis.
Chetomin induces degradation of XIAP and enhances TRAIL sensitivity in urogenital cancer cells.[Pubmed:21165560]
Int J Oncol. 2011 Feb;38(2):365-74.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is one of the most promising anti-cancer agents, but some tumor types develop resistance to TRAIL. Here, we report that Chetomin, an inhibitor of hypoxia-inducible factors, is a potent enhancer of TRAIL-induced apoptosis. TRAIL or Chetomin alone weakly induced apoptosis, but the combination of Chetomin and TRAIL synergistically induced apoptosis in prostate cancer PC-3 cells. The combination of Chetomin and TRAIL induces the activation of caspase-3, -8, -9 and -10. Among the apoptotic factors related to the TRAIL pathway, Chetomin markedly decreased the X-linked inhibitor of apoptosis (XIAP) protein levels in a dose-dependent manner, but other IAP family members, TRAIL receptors and Bcl-2 family members were not altered by Chetomin. Using XIAP siRNA instead of Chetomin, down-regulation of XIAP sensitized PC-3 cells to TRAIL-induced apoptosis. Conversely, transient transfection of XIAP reduced the apoptotic response to combined treatment with Chetomin and TRAIL. Treatment with Chetomin induced a rapid decrease in XIAP protein levels but had no effect on XIAP mRNA levels. Since Chetomin-mediated XIAP down-regulation was completely prevented by proteasome inhibitors, it was suggested that Chetomin induces the degradation of the XIAP protein in a proteasome-dependent manner. Additionally, Chetomin also sensitized renal cancer Caki-1 cells and bladder cancer UM-UC-3 cells to TRAIL-induced apoptosis via down-regulation of XIAP. Co-treatment of Chetomin and TRAIL did not enhance apoptosis in normal peripheral blood mononuclear cells (PBMC). Taken together, these findings suggest that TRAIL and Chetomin synergistically induce apoptosis in human urogenital cancer cells through a mechanism that involves XIAP down-regulation by Chetomin.
Studies on the Biosynthesis of Chetomin: Enantiospecific Synthesis of a Putative, Late-Stage Biosynthetic Intermediate.[Pubmed:24489414]
Tetrahedron. 2013 Jan 1;69(2):770-773.
The enantiospecific synthesis of desthioChetomin, a putative biosynthetic intermediate of the epidithiodioxopiperazine natural product Chetomin, is described. A diastereoselective N-alkylation was employed to form the key C3-N1' bond of the heterodimeric indoline core, followed by peptide coupling and dioxopiperazine cyclization with the requisite N-methyl amino acids. A related sarcosine-derived dioxopiperazine was prepared in the same manner. The first proposed biosynthesis of Chetomin is also detailed in the text.
Enhanced antitumor activity and mechanism of biodegradable polymeric micelles-encapsulated chetomin in both transgenic zebrafish and mouse models.[Pubmed:25175172]
Nanoscale. 2014 Oct 21;6(20):11940-52.
Chetomin is a promising molecule with anti-tumor activities in the epipolythiodioxopiperazine family of fungal secondary metabolites; however, strong hydrophobicity has limited its further applications. In this work, Chetomin was encapsulated into polymeric micelles to obtain an aqueous formulation, and the Chetomin loaded micelles (Che-M) exhibited small particle size and high encapsulation efficiency. When the concentration of copolymer was higher than the critical gelation concentration, the Che-M could form a thermosensitive hydrogel (Che-H), which was free-flowing sol at ambient temperature and converted into a non-flowing gel at body temperature. The molecular modeling study has indicated that Chetomin interacted with PCL as a core, which was embraced by PEG as a shell. Che-M showed equal cytotoxicity with free Chetomin, but the apoptosis inducing effects of Che-M were more significant. Besides, Che-M could increase the GSSG level, decrease the GSH level, and increase the ROS in CT26 cells. Furthermore, stronger inhibitory effects of Che-M were observed on embryonic angiogenesis, tumor-induced angiogenesis and tumor growth in transgenic zebrafish models. In addition, Che-M was effective in inhibiting tumor growth and prolonging survival in a subcutaneous CT26 tumor model. In a colorectal peritoneal carcinomatosis model, both Che-M and Che-H showed excellent therapeutic effects, but Che-H was more effective. In conclusion, Che-M and Che-H may serve as candidates for cancer therapy.


