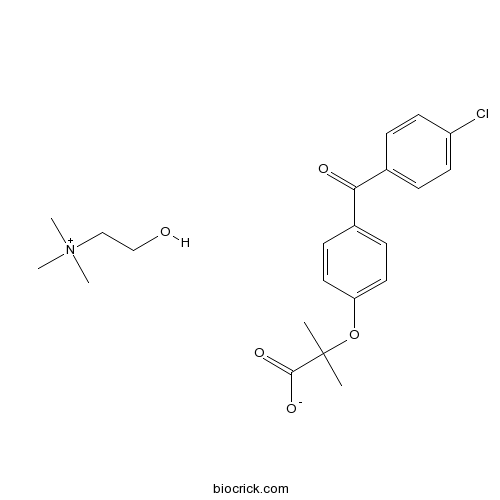
Choline FenofibrateCholine salt of fenofibric acid CAS# 856676-23-8 |
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Bay 65-1942 free base
Catalog No.:BCC1408
CAS No.:600734-02-9
- Bay 65-1942 HCl salt
Catalog No.:BCC1409
CAS No.:600734-06-3
- Bay 65-1942 R form
Catalog No.:BCC1410
CAS No.:758683-21-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 856676-23-8 | SDF | Download SDF |
| PubChem ID | 11350701 | Appearance | Powder |
| Formula | C22H28ClNO5 | M.Wt | 421.91 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ABT-335 | ||
| Solubility | Ethanol : 33.33 mg/mL (79.00 mM; Need ultrasonic) DMSO : 20 mg/mL (47.40 mM; Need ultrasonic) | ||
| Chemical Name | 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoate;2-hydroxyethyl(trimethyl)azanium | ||
| SMILES | CC(C)(C(=O)[O-])OC1=CC=C(C=C1)C(=O)C2=CC=C(C=C2)Cl.C[N+](C)(C)CCO | ||
| Standard InChIKey | JWAZHODZSADEHB-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C17H15ClO4.C5H14NO/c1-17(2,16(20)21)22-14-9-5-12(6-10-14)15(19)11-3-7-13(18)8-4-11;1-6(2,3)4-5-7/h3-10H,1-2H3,(H,20,21);7H,4-5H2,1-3H3/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Choline Fenofibrate (ABT-335) is the choline salt of fenofibric acid under clinical development as a combination therapy with rosuvastatin for the management of dyslipidemia.
IC50 value:
Target:
Several clinical trials have been developed with Choline Fenofibrate on Reverse Cholesterol Transport, Macular Edema and Hypertriglyceridemia. References: | |||||

Choline Fenofibrate Dilution Calculator

Choline Fenofibrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3702 mL | 11.8509 mL | 23.7017 mL | 47.4035 mL | 59.2543 mL |
| 5 mM | 0.474 mL | 2.3702 mL | 4.7403 mL | 9.4807 mL | 11.8509 mL |
| 10 mM | 0.237 mL | 1.1851 mL | 2.3702 mL | 4.7403 mL | 5.9254 mL |
| 50 mM | 0.0474 mL | 0.237 mL | 0.474 mL | 0.9481 mL | 1.1851 mL |
| 100 mM | 0.0237 mL | 0.1185 mL | 0.237 mL | 0.474 mL | 0.5925 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Choline Fenofibrate (ABT-335) is the choline salt of fenofibric acid under clinical development as a combination therapy with rosuvastatin for the management of dyslipidemia. Several clinical trials have been developed with Choline Fenofibrate on Reverse Cholesterol Transport, Macular Edema and Hypertriglyceridemia.
- Setiptiline maleate
Catalog No.:BCC1946
CAS No.:85650-57-3
- Asenapine
Catalog No.:BCC2476
CAS No.:85650-56-2
- Mirtazapine
Catalog No.:BCC4923
CAS No.:85650-52-8
- Laurycolactone B
Catalog No.:BCN3110
CAS No.:85643-77-2
- Laurycolactone A
Catalog No.:BCN3109
CAS No.:85643-76-1
- Curculigoside
Catalog No.:BCN4406
CAS No.:85643-19-2
- Boc-Ala-NH2
Catalog No.:BCC3046
CAS No.:85642-13-3
- WP1130
Catalog No.:BCC3686
CAS No.:856243-80-6
- Temozolomide
Catalog No.:BCC4386
CAS No.:85622-93-1
- (2-Aminoethyl)phosphinic acid
Catalog No.:BCN1761
CAS No.:85618-16-2
- Gynuramine
Catalog No.:BCN2085
CAS No.:85611-43-4
- Zaltidine
Catalog No.:BCC2068
CAS No.:85604-00-8
- CBiPES hydrochloride
Catalog No.:BCC7824
CAS No.:856702-40-4
- AM 114
Catalog No.:BCC3589
CAS No.:856849-35-9
- Tedizolid
Catalog No.:BCC1990
CAS No.:856866-72-3
- (-)-Blebbistatin
Catalog No.:BCC4375
CAS No.:856925-71-8
- alpha-Conidendrin
Catalog No.:BCN4407
CAS No.:85699-62-3
- (3S,3'R,8R,9R,9As)-8-methoxy-3'-methyl-3-[(2S,4S)-4-methyl-5-oxooxolan-2-yl]spiro[1,2,3,5,6,7,8,9a-octahydropyrrolo[1,2-a]azepine-9,5'-oxolane]-2'-one
Catalog No.:BCC9250
CAS No.:85700-47-6
- Scopine HCl
Catalog No.:BCC4940
CAS No.:85700-55-6
- WP1066
Catalog No.:BCC2194
CAS No.:857064-38-1
- TMC353121
Catalog No.:BCC2004
CAS No.:857066-90-1
- PF 915275
Catalog No.:BCC7631
CAS No.:857290-04-1
- 3-Hydroxysarpagine
Catalog No.:BCN4566
CAS No.:857297-90-6
- Isoflavidinin
Catalog No.:BCN7604
CAS No.:85734-02-7
Absolute oral bioavailability of fenofibric acid and choline fenofibrate in rats determined by ultra-performance liquid chromatography tandem mass spectrometry.[Pubmed:27594083]
Biomed Chromatogr. 2017 Apr;31(4).
Choline Fenofibrate is the choline salt of fenofibric acid, which releases free fenofibric acid in the gastrointestinal tract. To estimate the absolute oral bioavailability of fenofibric acid and Choline Fenofibrate, a novel and sensitive UPLC-MS/MS method with liquid-liquid extraction procedure was developed for the determination of fenofibric acid in rat plasma. The separation was achieved on a Phenomenex Kinetex C18 column (50 x 2.1 mm, 2.6 mum) containing 2 mm ammonium acetate-methanol with a gradient elution program. Validations of this method including specificity, sensitivity (limit of quantification, 5 ng/mL), linearity (0.005-10 mug/mL), accuracy (within +/-4.3%), precision (intra- and inter-day coefficient of variation <11.3%), recovery (94.9-105.2% for fenofibric acid), matrix effect, stability and dilution, were all within acceptable limits. This method successfully supported the determination of fenofibric acid and Choline Fenofibrate. The absolute oral bioavailability was 93.4% for Choline Fenofibrate and 40.0% for fenofibric acid. These results suggested that Choline Fenofibrate and fenofibric acid had a better in vivo pharmacokinetic behavior than that of fenofibrate. The two new orally administrated pharmaceuticals, fenofibric acid and Choline Fenofibrate, can be developed as alternatives to fenofibrate.
Comparison of efficacy and safety of choline fenofibrate (fenofibric acid) to micronized fenofibrate in patients of mixed dyslipidemia: A randomized, open-label, multicenter clinical trial in Indian population.[Pubmed:26904471]
Indian J Endocrinol Metab. 2016 Jan-Feb;20(1):67-71.
INTRODUCTION: Choline Fenofibrate is a newly developed choline salt of fenofibric acid, which is more hydrophilic than fenofibrate. This study was initiated to evaluate the safety and efficacy of Choline Fenofibrate in comparison to micronized fenofibrate among Indian patients of mixed dyslipidemia. METHODS: A multicenter, open-label, randomized, active controlled, comparative, parallel group study was conducted at around 10 centers spread all across the country. Mixed dyslipidemia patients (serum triglycerides [TG] levels between 150 and 500 mg/dl), aged 18-70 years and taking stable statin dose for 8 weeks were randomized to Choline Fenofibrate 135 mg delayed release tablets and micronized fenofibrate 160 mg tablets once daily for 12 weeks. The primary endpoint of the study was percentage change in serum TG level at the end of 12 weeks. RESULTS: A total of 226 patients were enrolled in this study, of which 116 patients were administered Choline Fenofibrate and 110 patients were administered micronized fenofibrate. At the end of 12 weeks, there was a significant reduction in TG level (34.24% in Choline Fenofibrate group and 38.13% reduction in micronized fenofibrate group). However, the difference between group was not statistically different (P = 0.471). Similarly, there was a significant increase in high-density lipoprotein cholesterol at the end of 12 weeks (10% increase in Choline Fenofibrate group and 9% increase in micronized fenofibrate group); but the difference between the group was not statistically significant (P = 0.598). Both the treatment was safe and well tolerated. CONCLUSION: Choline Fenofibrate delayed release 135 mg is as safe and effective as 160 mg of micronized fenofibrate in Indian patients with mixed dyslipidemia.
Novel fenofibric acid-loaded controlled release pellet bioequivalent to choline fenofibrate-loaded commercial product in beagle dogs.[Pubmed:26024820]
Int J Pharm. 2015 Jul 25;490(1-2):273-80.
The objective of this study was to develop a novel fenofibric acid-loaded controlled release pellet showing enhanced, or equivalent to, bioavailability compared with two commercially available products containing fenofibrate or Choline Fenofibrate. The effect of solubilizing agents on drug solubility and the impact of fillers on core properties were investigated. Among them, magnesium carbonate most improved drug solubility, and kappa-carrageenan provided the best spherical cores. The fenofibric acid-loaded pellet was prepared with magnesium carbonate and kappa-carrageenan employing the extrusion/spheronizing technique followed by coating with ethylcellulose. Furthermore, dissolution and pharmacokinetic study in beagle dogs were performed compared to the fenofibrate-loaded commercial tablet (FCT) and Choline Fenofibrate-loaded commercial mini-tablet (CFCM). This fenofibric acid-loaded pellet showed controlled release of the drug in phosphate buffer (pH 6.8) and 0.025 M sodium laurylsulfate within 4h. Furthermore, this pellet and CFCM exhibited similar dissolution profiles. Plasma concentrations greater than 1,000 ng/ml were maintained from 30 min to 8h, suggesting a sustained release pattern. Also, the fenofibric acid-loaded pellet gave significantly higher AUC and Cmax values than FCT, indicating that it improved the bioavailability of fenofibrate due to enhanced solubility and sustained release. In addition, this pellet and CFCM were not significantly different in terms of pharmacokinetic parameters including AUC, Cmax and Tmax. Thus, this pellet was bioequivalent to CFCM in beagle dogs. In conclusion, this fenofibric acid-loaded controlled release pellet would be a potential alternative to the Choline Fenofibrate-loaded commercial product.


