Dynole 34-2Dynamin I inhibitor CAS# 1128165-88-7 |
- ML-7 hydrochloride
Catalog No.:BCC1770
CAS No.:110448-33-4
- TAK-438
Catalog No.:BCC1182
CAS No.:1260141-27-2
- Istaroxime
Catalog No.:BCC1660
CAS No.:203737-93-3
- Istaroxime hydrochloride
Catalog No.:BCC1661
CAS No.:374559-48-5
- 20-HETE
Catalog No.:BCC1301
CAS No.:79551-86-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1128165-88-7 | SDF | Download SDF |
| PubChem ID | 44157463 | Appearance | Powder |
| Formula | C25H36N4O | M.Wt | 408.58 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 50 mM in ethanol | ||
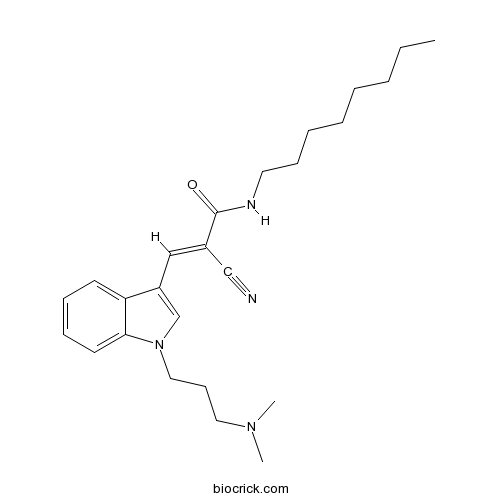
| Chemical Name | (E)-2-cyano-3-[1-[3-(dimethylamino)propyl]indol-3-yl]-N-octylprop-2-enamide | ||
| SMILES | CCCCCCCCNC(=O)C(=CC1=CN(C2=CC=CC=C21)CCCN(C)C)C#N | ||
| Standard InChIKey | MYIMRONQBLZJFI-DYTRJAOYSA-N | ||
| Standard InChI | InChI=1S/C25H36N4O/c1-4-5-6-7-8-11-15-27-25(30)21(19-26)18-22-20-29(17-12-16-28(2)3)24-14-10-9-13-23(22)24/h9-10,13-14,18,20H,4-8,11-12,15-17H2,1-3H3,(H,27,30)/b21-18+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dynamin I inhibitor (IC50 = 1.3 μM). Inhibits receptor-mediated endocytosis (RME) (IC50 = 5.0 μM). |

Dynole 34-2 Dilution Calculator

Dynole 34-2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4475 mL | 12.2375 mL | 24.475 mL | 48.95 mL | 61.1875 mL |
| 5 mM | 0.4895 mL | 2.4475 mL | 4.895 mL | 9.79 mL | 12.2375 mL |
| 10 mM | 0.2448 mL | 1.2238 mL | 2.4475 mL | 4.895 mL | 6.1188 mL |
| 50 mM | 0.049 mL | 0.2448 mL | 0.4895 mL | 0.979 mL | 1.2238 mL |
| 100 mM | 0.0245 mL | 0.1224 mL | 0.2448 mL | 0.4895 mL | 0.6119 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid
Catalog No.:BCC8464
CAS No.:112811-72-0
- 1-Cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid ethyl ester
Catalog No.:BCC8465
CAS No.:112811-71-9
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Letrozole
Catalog No.:BCC1063
CAS No.:112809-51-5
- Osthenone
Catalog No.:BCN4731
CAS No.:112789-90-9
- Clemastanin B
Catalog No.:BCC8152
CAS No.:112747-98-5
- Galanin (1-15) (porcine, rat)
Catalog No.:BCC5762
CAS No.:112747-70-3
- exo-IWR 1
Catalog No.:BCC7823
CAS No.:1127442-87-8
- IWR-1-endo
Catalog No.:BCC5102
CAS No.:1127442-82-3
- Oleonuezhenide
Catalog No.:BCN6011
CAS No.:112693-21-7
- Erigeside C
Catalog No.:BCN6010
CAS No.:112667-09-1
- Fragransin A2
Catalog No.:BCN6008
CAS No.:112652-46-7
- Methyllycaconitine citrate
Catalog No.:BCC6897
CAS No.:112825-05-5
- 24R-Calcipotriol
Catalog No.:BCC1304
CAS No.:112827-99-3
- (24R)-MC 976
Catalog No.:BCC1289
CAS No.:112828-09-8
- (24S)-MC 976
Catalog No.:BCC1291
CAS No.:112849-14-6
- Fmoc-D-Met-OH
Catalog No.:BCC3532
CAS No.:112883-40-6
- Fmoc-D-Nle-OH
Catalog No.:BCC3300
CAS No.:112883-41-7
- Fmoc-N-Me-Nle-OH
Catalog No.:BCC3299
CAS No.:112883-42-8
- Fmoc-2-Nal-OH
Catalog No.:BCC3292
CAS No.:112883-43-9
- Mosapride
Catalog No.:BCC4078
CAS No.:112885-41-3
- Mosapride Citrate
Catalog No.:BCC1065
CAS No.:112885-42-4
- Raltitrexed
Catalog No.:BCC4457
CAS No.:112887-68-0
- BzATP triethylammonium salt
Catalog No.:BCC7643
CAS No.:112898-15-4
Synthesis of Dynole 34-2, Dynole 2-24 and Dyngo 4a for investigating dynamin GTPase.[Pubmed:24651498]
Nat Protoc. 2014 Apr;9(4):851-70.
Dynamin is a large GTPase with roles in membrane fission during clathrin-mediated endocytosis, in actin dynamics and in cytokinesis. Defects in dynamin have been linked to human diseases. The synthesis of a dynamin modulator toolkit comprising two different inhibitor classes is described. The first series comprises Dynole 34-2, Dynole 2-24 and the inactive control Dynole 31-2. The Dynole compounds act on the dynamin G domain, are not GTP competitive and can be synthesized in 2-3 d. Knoevenagel condensation of 1-(3-(dimethylamino)propyl)-1H-indole-3-carbaldehyde (1) with cyanoamides (2 and 3) affords Dynole 31-2 and Dynole 34-2, respectively. Reductive amination of 1 with decylamine gives Dynole 2-24. The second series acts at an allosteric site in the G domain of dynamin and comprises Dyngo 4a and Dyngo O (inactive control). Both are synthesized in an overnight reaction via condensation of 3-hydroxy-2-naphthoic hydrazide with 2,4,5-trihydroxybenzaldehyde to afford Dyngo 4a, or with benzaldehyde to afford Dyngo O.
Inhibition of dynamin by dynole 34-2 induces cell death following cytokinesis failure in cancer cells.[Pubmed:21750222]
Mol Cancer Ther. 2011 Sep;10(9):1553-62.
Inhibitors of mitotic proteins such as Aurora kinase and polo-like kinase have shown promise in preclinical or early clinical development for cancer treatment. We have reported that the MiTMAB class of dynamin small molecule inhibitors are new antimitotic agents with a novel mechanism of action, blocking cytokinesis. Here, we examined 5 of the most potent of a new series of dynamin GTPase inhibitors called dynoles. They all induced cytokinesis failure at the point of abscission, consistent with inhibition of dynamin while not affecting other cell cycle stages. All 5 dynoles inhibited cell proliferation (MTT and colony formation assays) in 11 cancer cell lines. The most potent GTPase inhibitor, Dynole 34-2, also induced apoptosis, as revealed by cell blebbing, DNA fragmentation, and PARP cleavage. Cell death was induced specifically following cytokinesis failure, suggesting that Dynole 34-2 selectively targets dividing cells. Dividing HeLa cells were more sensitive to the antiproliferative properties of all 5 dynoles compared with nondividing cells, and nontumorigenic fibroblasts were less sensitive to cell death induced by Dynole 34-2. Thus, the dynoles are a second class of dynamin GTPase inhibitors, with Dynole 34-2 as the lead compound, that are novel antimitotic compounds acting specifically at the abscission stage.
Iminochromene inhibitors of dynamins I and II GTPase activity and endocytosis.[Pubmed:20426422]
J Med Chem. 2010 May 27;53(10):4094-102.
Herein we report the synthesis of discrete iminochromene ("iminodyn") libraries (14-38) as potential inhibitors of dynamin GTPase. Thirteen iminodyns were active (IC(50) values of 260 nM to 100 microM), with N,N-(ethane-1,2-diyl)bis(7,8-dihydroxy-2-iminochromene-3-carboxamide) (17), N,N-(ethane-1,2-diyl)bis(7,8-dihydroxy-2-iminochromene-3-carboxamide) (22), and N,N-(ethane-1,2-diyl)bis(7,8-dihydroxy-2-iminochromene-3-carboxamide) (23) (IC(50) values of 330 +/- 70, 450 +/- 50, and 260 +/- 80 nM, respectively) being the most potent. Five of the most potent iminodyns all inhibited dynamins I and II approximately equally. Iminodyn-22 displayed uncompetitive inhibition with respect to GTP. Selected iminodyns were evaluated for their ability to block receptor mediated endocytosis (RME, mediated by dynamin II) and synaptic vesicle endocytosis (SVE, mediated by dynamin I), with 17 showing no activity while 22 returned RME and SVE IC(50) values of 10.7 +/- 4.5 and 99.5 +/- 1.7 microM, respectively. The iminodyns reported herein represent a new chemical class of the first nanomolar potent dynamin inhibitors that are also effective endocytosis inhibitors.
Inhibition of dynamin mediated endocytosis by the dynoles--synthesis and functional activity of a family of indoles.[Pubmed:19459681]
J Med Chem. 2009 Jun 25;52(12):3762-73.
Screening identified two bisindolylmaleimides as 100 microM inhibitors of the GTPase activity of dynamin I. Focused library approaches allowed development of indole-based dynamin inhibitors called dynoles. 100-Fold in vitro enhancement of potency was noted with the best inhibitor, 2-cyano-3-(1-(2-(dimethylamino)ethyl)-1H-indol-3-yl)-N-octylacrylamide (Dynole 34-2), a 1.3 +/- 0.3 microM dynamin I inhibitor. Dynole 34-2 potently inhibited receptor mediated endocytosis (RME) internalization of Texas red-transferrin. The rank order of potency for a variety of dynole analogues on RME in U2OS cells matched their rank order for dynamin inhibition, suggesting that the mechanism of inhibition is via dynamin. Dynoles are the most active dynamin I inhibitors reported for in vitro or RME evaluations. Dynole 34-2 is 15-fold more active than dynasore against dynamin I and 6-fold more active against dynamin mediated RME (IC(50) approximately 15 microM; RME IC(50) approximately 80 microM). The dynoles represent a new series of tools to better probe endocytosis and dynamin-mediated trafficking events in a variety of cells.


