EfinaconazoleCAS# 164650-44-6 |
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Nelfinavir
Catalog No.:BCC4138
CAS No.:159989-64-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Tenofovir hydrate
Catalog No.:BCC4261
CAS No.:206184-49-8
- Dapivirine (TMC120)
Catalog No.:BCC3882
CAS No.:244767-67-7
- Zidovudine
Catalog No.:BCC5024
CAS No.:30516-87-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
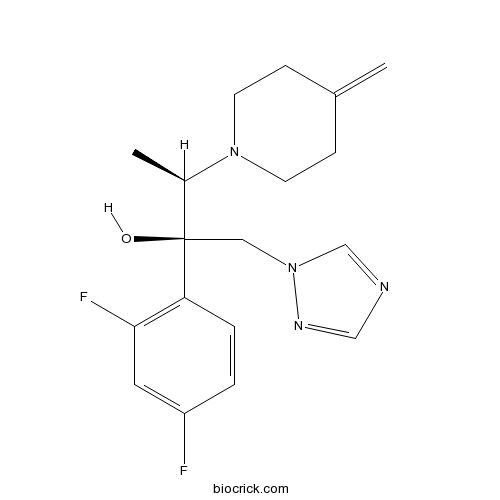
| Cas No. | 164650-44-6 | SDF | Download SDF |
| PubChem ID | 489181 | Appearance | Powder |
| Formula | C18H22F2N4O | M.Wt | 348.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (287.03 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2R,3R)-2-(2,4-difluorophenyl)-3-(4-methylidenepiperidin-1-yl)-1-(1,2,4-triazol-1-yl)butan-2-ol | ||
| SMILES | CC(C(CN1C=NC=N1)(C2=C(C=C(C=C2)F)F)O)N3CCC(=C)CC3 | ||
| Standard InChIKey | NFEZZTICAUWDHU-RDTXWAMCSA-N | ||
| Standard InChI | InChI=1S/C18H22F2N4O/c1-13-5-7-23(8-6-13)14(2)18(25,10-24-12-21-11-22-24)16-4-3-15(19)9-17(16)20/h3-4,9,11-12,14,25H,1,5-8,10H2,2H3/t14-,18-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Efinaconazole Dilution Calculator

Efinaconazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8703 mL | 14.3517 mL | 28.7035 mL | 57.4069 mL | 71.7587 mL |
| 5 mM | 0.5741 mL | 2.8703 mL | 5.7407 mL | 11.4814 mL | 14.3517 mL |
| 10 mM | 0.287 mL | 1.4352 mL | 2.8703 mL | 5.7407 mL | 7.1759 mL |
| 50 mM | 0.0574 mL | 0.287 mL | 0.5741 mL | 1.1481 mL | 1.4352 mL |
| 100 mM | 0.0287 mL | 0.1435 mL | 0.287 mL | 0.5741 mL | 0.7176 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Aldicarb sulfone
Catalog No.:BCC5476
CAS No.:1646-88-4
- KPT-9274
Catalog No.:BCC6461
CAS No.:1643913-93-2
- Immepip dihydrobromide
Catalog No.:BCC6853
CAS No.:164391-47-3
- Boc-D-Phe(4-NH2)-OH
Catalog No.:BCC3155
CAS No.:164332-89-2
- Phosphoramidon Disodium Salt
Catalog No.:BCC5484
CAS No.:164204-38-0
- AM630
Catalog No.:BCC1353
CAS No.:164178-33-0
- LY 311727
Catalog No.:BCC7728
CAS No.:164083-84-5
- Clerodenoside A
Catalog No.:BCN1725
CAS No.:164022-75-7
- Longiferone B
Catalog No.:BCN7378
CAS No.:1639810-67-5
- Kaempferol-3-O-(6''-O-cis-coumaryl)glucoside
Catalog No.:BCN1538
CAS No.:163956-16-9
- 12E,14-Labdadien-20,8beta-olide
Catalog No.:BCN7395
CAS No.:1639257-37-6
- 8(17),12E,14-Labdatrien-20-oic acid
Catalog No.:BCN7396
CAS No.:1639257-36-5
- Dutasteride
Catalog No.:BCC1097
CAS No.:164656-23-9
- CGP60474
Catalog No.:BCC1474
CAS No.:164658-13-3
- 4-(3,4-Dimethoxyphenyl)-3-butene-1,2-diol
Catalog No.:BCN1537
CAS No.:164661-12-5
- Podophyllotoxin 4-O-glucoside
Catalog No.:BCN8064
CAS No.:16481-54-2
- G-5555
Catalog No.:BCC8095
CAS No.:1648863-90-4
- Azaperone
Catalog No.:BCC4630
CAS No.:1649-18-9
- ML352
Catalog No.:BCC6434
CAS No.:1649450-12-3
- N9-Methylharman
Catalog No.:BCN3368
CAS No.:16498-64-9
- Calyxin B
Catalog No.:BCN1726
CAS No.:164991-53-1
- Kukoamine B
Catalog No.:BCN3836
CAS No.:164991-67-7
- Decumbenine B
Catalog No.:BCC8313
CAS No.:164991-68-8
- Orcinol gentiobioside
Catalog No.:BCN2815
CAS No.:164991-86-0
Efinaconazole 10% and Tavaborole 5% Penetrate Across Poly-ureaurethane 16%: Results of In Vitro Release Testing and Clinical Implications of Onychodystrophy in Onychomycosis.[Pubmed:27602975]
J Drugs Dermatol. 2016 Sep 1;15(9):1116-20.
BACKGROUND: Poly-ureaurethane has been previously described for the management of dry, brittle, and in general, dystrophic nails. The polymer yields a waterproof, breathable barrier to protect the nail plate and prevent further damage to the nail, while regulating transonychial water loss (TOWL). Because nail dystrophy and dessication are contributing factors to onychomycosis, a barrier that protects the nail but also allows a topical antifungal to permeate its shield is potentially an advantageous combination. Oral antifungals such as terbinafine, itraconazole, and fluconazole, as well as the newer topical antifungals Efinaconazole and tavaborole (although formulated to penetrate the nail unit and work with the porosity and inherent electrical charge of the nail plate), do not take into account nail damage that has been created from years of harboring a dermatophyte infection. Up to 50% of cases presumed to be onychomycosis are in fact onychodystrophy without fungal infection, and laboratory testing for fungus should be obtained prior to initiating antifungal treatment. Whether a nail has onychomycosis, or onychodystrophy due to other causes, barrier function and structural integrity are compromised in diseased nails, and should be addressed. A poly-ureaurethane barrier that protects against wetting/drying, fungal reservoirs, and microtrauma, followed by the addition of oral or topical antifungals after laboratory fungal confirmation may optimize outcomes in the treatment of onychomycosis.
OBJECTIVE: The purpose of this work was to determine through in vitro release testing (IVRT) whether poly-ureaurethane 16% allows for penetration of Efinaconazole 10% or tavaborole 5%. Results could spur subsequent clinical studies which would have implications for the addition of an antifungal based on fungal confirmation, after addresssing the underlying nail dystrophy primarily.
METHODS: A vertical diffusion cell system was used to evaluate the ability of Efinaconazole 10% and tavaborole 5% to penetrate across poly-ureaurethane 16%. The diffusion cells had a 1.0 cm2 surface area and approximately 8 mL receptor volume. Poly-ureaurethane 16% was applied to a 0.45 μm nylon membrane and allowed to dry before use. Efinaconazole 10% or tavaborole 5% was then applied to the poly-ureaurethane 16% coated membrane, and samples were pulled from the receptor chamber at various times. Reverse phase chromatography was then used to assess the penetration of each active ingredient across the membrane.
RESULTS: The flux and permeability of Efinaconazole or tavaborole across poly-ureaurethane 16% were determined from Efinaconazole 10% or tavaborole 5%, respectively. The flux and permeability of Efinaconazole were determined to be 503.9 +/- 31.9 μg/cm2/hr and 14.0 +/- 0.9 nm/sec. The flux and permeability of tavaborole were determined to be 755.5 +/- 290.4 μg/cm2/hr and 42.0 +/- 16.1 nm/sec.
CONCLUSION: In addition to the treatment of onychoschizia, onychorrhexis, and other signs of severe dessication of the nail plate, a barrier that regulates TOWL should be considered in the management onychomycosis to address barrier dysfunction and to promote stabilization of the damaged nail. Previously published flux values across the nail are reported to be 1.4 μg/cm2/day for Efinaconazole and 204 μg/cm2/day for tavaborole. These values are substantially lower than the herein determined flux for both molecules across poly-ureaurethane 16%. A comparison of the data suggests that poly-ureaurethane 16%, if used prior to Efinaconazole or tavaborole, would not limit the ability of either active ingredient to access the nail, and therefore, would be unlikely to reduce their antifungal effect. Onychodystrophy is inherent in, and often precedes onychomycosis, and consideration should be given for initiation of treatment in the same sequence: stabilizing and protecting the nail plate barrier primarily, and subsequently adding oral or topical antifungals after laboratory confirmation. Future clinical studies will be needed to determine combination efficacy for in vivo use.
J Drugs Dermatol. 2016;15(9):1116-1120.
Update on Efinaconazole 10% Topical Solution for the Treatment of Onychomycosis.[Pubmed:27825175]
Skin Therapy Lett. 2016 Nov;21(6):7-11.
Efinaconazole 10% nail solution is a novel topical antifungal drug for the treatment of onychomycosis. Two Phase III trials were completed using Efinaconazole 10% nail solution, where 17.8% and 15.2% of patients achieved complete cure, and 55.2% and 53.4% achieved mycological cure. Several post hoc analyses were carried out using data from Phase III trials to determine the efficacy of Efinaconazole with respect to disease duration, disease progression, and comorbidities of diabetes or tinea pedis with onychomycosis. Efinaconazole produced higher efficacy rates with patients presenting onychomycosis in a small portion of the toenail (Efinaconazole increased from 16.1% to 29.4%, suggesting combination therapy improved results. Most interestingly, there was no difference in Efinaconazole efficacy between diabetic and non-diabetic groups, indicating Efinaconazole could be a safe and effective form of treatment for diabetics. Overall, Efinaconazole 10% nail solution shows potential as an antifungal therapy for the treatment of onychomycosis.


