TenofovirHIV reverse transcriptase inhibitor CAS# 147127-20-6 |
- Emtricitabine
Catalog No.:BCC3774
CAS No.:143491-57-0
- Tenofovir Disoproxil Fumarate
Catalog No.:BCC1108
CAS No.:202138-50-9
- Entecavir Hydrate
Catalog No.:BCC1109
CAS No.:209216-23-9
- Lersivirine
Catalog No.:BCC1698
CAS No.:473921-12-9
- Didanosine
Catalog No.:BCC3763
CAS No.:69655-05-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
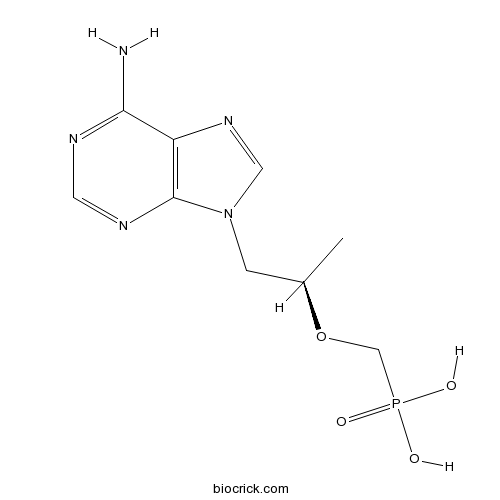
| Cas No. | 147127-20-6 | SDF | Download SDF |
| PubChem ID | 464205 | Appearance | Powder |
| Formula | C9H14N5O4P | M.Wt | 287.21 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | PMPA, (<em>R</em>)-9-(2-Phosphonoylmethoxypropyl)adenine | ||
| Solubility | H2O : 2 mg/mL (6.96 mM; Need ultrasonic) | ||
| Chemical Name | [(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethylphosphonic acid | ||
| SMILES | CC(CN1C=NC2=C1N=CN=C2N)OCP(=O)(O)O | ||
| Standard InChIKey | SGOIRFVFHAKUTI-ZCFIWIBFSA-N | ||
| Standard InChI | InChI=1S/C9H14N5O4P/c1-6(18-5-19(15,16)17)2-14-4-13-7-8(10)11-3-12-9(7)14/h3-4,6H,2,5H2,1H3,(H2,10,11,12)(H2,15,16,17)/t6-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selectively inhibits HIV reverse transcriptase (RNA-dependent DNA polymerase). Prevents cytotoxicity in SIV-infected C-8166 cells in vitro (IC50 = 1.5 μM). Antiviral agent. |

Tenofovir Dilution Calculator

Tenofovir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4818 mL | 17.4089 mL | 34.8177 mL | 69.6355 mL | 87.0443 mL |
| 5 mM | 0.6964 mL | 3.4818 mL | 6.9635 mL | 13.9271 mL | 17.4089 mL |
| 10 mM | 0.3482 mL | 1.7409 mL | 3.4818 mL | 6.9635 mL | 8.7044 mL |
| 50 mM | 0.0696 mL | 0.3482 mL | 0.6964 mL | 1.3927 mL | 1.7409 mL |
| 100 mM | 0.0348 mL | 0.1741 mL | 0.3482 mL | 0.6964 mL | 0.8704 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Selectively inhibits HIV reverse transcriptase (RNA-dependent DNA polymerase). Prevents cytotoxicity in SIV-infected C-8166 cells in vitro (IC50 = 1.5 μM). Antiviral agent.
- 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidinyl-5-yl-formyl
Catalog No.:BCC8651
CAS No.:147118-37-4
- Methyl (3R)-3-(tert-butyldimethylsilyloxy)-5-oxo-6-triphenylphosphoranylidenehexanoate
Catalog No.:BCC9031
CAS No.:147118-35-2
- Maropitant
Catalog No.:BCC1728
CAS No.:147116-67-4
- Rosuvastatin Calcium
Catalog No.:BCC3853
CAS No.:147098-20-2
- 5,6-Dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-4-one
Catalog No.:BCC8722
CAS No.:147086-79-1
- Alcaftadine
Catalog No.:BCC5260
CAS No.:147084-10-4
- Trovafloxacin mesylate
Catalog No.:BCC3931
CAS No.:147059-75-4
- Rocaglaol
Catalog No.:BCN1653
CAS No.:147059-46-9
- Cyclo(Phe-Pro)
Catalog No.:BCN2416
CAS No.:14705-60-3
- MK591
Catalog No.:BCC1766
CAS No.:147030-01-1
- Menthyl-5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-[1,3]oxathiolane-2-carboxylic acid
Catalog No.:BCC9019
CAS No.:147027-10-9
- 3'-O-Demethylarctigenin
Catalog No.:BCN3544
CAS No.:147022-95-5
- 4-Chloro-L-phenylalanine Hydrochloride
Catalog No.:BCC2638
CAS No.:123053-23-6
- TT 232
Catalog No.:BCC6248
CAS No.:147159-51-1
- N-6-Methyl-7,7-dioxo-2-sulfamoyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-yl]acetamide
Catalog No.:BCC9077
CAS No.:147200-03-1
- Arecaidine propargyl ester tosylate
Catalog No.:BCC6628
CAS No.:147202-94-6
- Delavirdine mesylate
Catalog No.:BCC4069
CAS No.:147221-93-0
- glatiramer acetate
Catalog No.:BCC5642
CAS No.:147245-92-9
- 7ACC2
Catalog No.:BCC5554
CAS No.:1472624-85-3
- KRCA 0008
Catalog No.:BCC8007
CAS No.:1472795-20-2
- Antibiotic PF 1052
Catalog No.:BCN1828
CAS No.:147317-15-5
- Cylindramide
Catalog No.:BCN1832
CAS No.:147362-39-8
- MKT 077
Catalog No.:BCC6241
CAS No.:147366-41-4
- Ligupurpuroside A
Catalog No.:BCC8198
CAS No.:147396-01-8
Monotherapy with tenofovir disoproxil fumarate for multiple drug-resistant chronic hepatitis B: 3-year trial.[Pubmed:28370419]
Hepatology. 2017 Sep;66(3):772-783.
Combination therapy has been recommended for the treatment of patients harboring multiple drug-resistant hepatitis B virus (HBV). However, we recently demonstrated that monotherapy with Tenofovir disoproxil fumarate (TDF) for 48 weeks displayed noninferior efficacy to TDF plus entecavir (ETV) combination therapy in patients with HBV resistant to multiple drugs, including ETV and adefovir. Nonetheless, whether prolonged TDF monotherapy would be safe and increase the virologic response rate in these patients was unclear. Among 192 patients with HBV-resistance mutations to ETV and/or adefovir, who were randomized to receive TDF monotherapy (n = 95) or TDF/ETV combination therapy (n = 97) for 48 weeks, 189 agreed to continue TDF monotherapy (TDF-TDF group) or to switch to TDF monotherapy (TDF/ETV-TDF group) and 180 (93.8%) completed the 144-week study. Serum HBV DNA <15 IU/mL at week 48, the primary efficacy endpoint, was achieved in 66.3% in the TDF-TDF group and 68.0% in the TDF/ETV-TDF group (P = 0.80). At week 144, the proportion with HBV DNA <15 IU/mL increased to 74.5%, which was significantly higher compared with that at week 48 (P = 0.03), without a significant difference between groups (P = 0.46). By on-treatment analysis, a total of 79.4% had HBV DNA <15 IU/mL at week 144. Transient virologic breakthrough occurred in 6 patients, which was due to poor drug adherence. At week 144, 19 patients who had HBV DNA levels >60 IU/mL qualified for genotypic resistance analysis, and 6 retained some of their baseline resistance mutations of HBV. No patients developed additional resistance mutations throughout the study period. CONCLUSION: TDF monotherapy was efficacious and safe for up to 144 weeks, providing an increasing rate of virologic response in heavily pretreated patients with multidrug-resistant HBV. (Hepatology 2017;66:772-783).
Single-dose pharmacokinetics of tenofovir alafenamide and its active metabolite in the mucosal tissues.[Pubmed:28369415]
J Antimicrob Chemother. 2017 Jun 1;72(6):1731-1740.
Objectives: Tenofovir alafenamide, a prodrug of Tenofovir, produces higher PBMC concentrations of Tenofovir diphosphate (Tenofovir-dp) than Tenofovir disoproxil fumarate. To understand Tenofovir alafenamide's mucosal tissue distribution and its implications for pre-exposure prophylaxis, we characterized Tenofovir-dp in female genital tract (FGT) and lower gastrointestinal (GI) tissues. Methods: Healthy seronegative women were given 5, 10 or 25 mg of Tenofovir alafenamide ( n = 8/group). Each participant provided plasma, PBMC and cervical, vaginal and rectal tissue samples over 14 days. Plasma, cell lysate and tissue homogenate concentrations were analysed by LC-MS/MS. Dose proportionality was declared in plasma and PBMCs if the natural log AUC versus natural log dose regression line 90% CI was within 0.57-1.43. In vitro Tenofovir-dp formation was assessed in PBMCs and ectocervical (Ect1/E6E7) and vaginal (VK2/E6E7) cells incubated in 0.5 and 10 muM Tenofovir alafenamide or Tenofovir. clinicaltrials.gov: NCT02357602. Results: Following single doses of 5, 10 and 25 mg, median (IQR) Tenofovir plasma AUC 0-14 days was 52.8 (49.5-59.6), 78.1 (68.2-86.9) and 169.7 (131.2-211.4) ng.h/mL and Tenofovir-dp PBMC AUC 0-14 days was 2268 (1519-4090), 4584 (3113-5734) and 9306 (6891-10785) fmol.h/10 6 cells, respectively. Tenofovir was quantifiable in 52% and 92% of FGT and GI tissues, whereas Tenofovir-dp was quantifiable in only 5% and 19% of FGT and GI tissues, respectively. Plasma Tenofovir and PBMC Tenofovir-dp were dose proportional (90% CI = 0.87-1.15 and 0.62-1.02, respectively). In vitro Tenofovir-dp was 1.7-17-fold higher in epithelial cells than PBMCs. Conclusions: After Tenofovir alafenamide dosing in vivo , Tenofovir-dp was unquantifiable in most tissues (91%) although cervical and vaginal epithelial cells efficiently formed Tenofovir-dp from Tenofovir alafenamide in vitro . These findings warrant further investigation of Tenofovir alafenamide's pharmacology.
Tenofovir Inhibits Wound Healing of Epithelial Cells and Fibroblasts from the Upper and Lower Human Female Reproductive Tract.[Pubmed:28368028]
Sci Rep. 2017 Apr 3;8:45725.
Disruption of the epithelium in the female reproductive tract (FRT) is hypothesized to increase HIV infection risk by interfering with barrier protection and facilitating HIV-target cell recruitment. Here we determined whether Tenofovir (TFV), used vaginally in HIV prevention trials, and Tenofovir alafenamide (TAF), an improved prodrug of TFV, interfere with wound healing in the human FRT. TFV treatment of primary epithelial cells and fibroblasts from the endometrium (EM), endocervix (CX) and ectocervix (ECX) significantly delayed wound closure. Reestablishment of tight junctions was compromised in EM and CX epithelial cells even after wound closure occurred. In contrast, TAF had no inhibitory effect on wound closure or tight junction formation following injury. TAF accumulated inside genital epithelial cells as TFV-DP, the active drug form. At elevated levels of TAF treatment to match TFV intracellular TFV-DP concentrations, both equally impaired barrier function, while wound closure was more sensitive to TFV. Furthermore, TFV but not TAF increased elafin and MIP3a secretion following injury, molecules known to be chemotactic for HIV-target cells. Our results highlight the need of evaluating antiretroviral effects on genital wound healing in future clinical trials. A possible link between delayed wound healing and increased risk of HIV acquisition deserves further investigation.
Bone mineral density reductions after tenofovir disoproxil fumarate initiation and changes in phosphaturia: a secondary analysis of ACTG A5224s.[Pubmed:28369419]
J Antimicrob Chemother. 2017 Jul 1;72(7):2042-2048.
Background: It is unknown if the greater reductions in bone mineral density (BMD) associated with initiation of Tenofovir disoproxil fumarate compared with abacavir in previously untreated HIV-infected participants in the ACTG A5224s clinical trial were associated with potentially worsening Tenofovir-related phosphaturia. Methods: We correlated changes in BMD at the hip and spine with changes in phosphaturia [transtubular reabsorption of phosphorus (TRP) and tubular maximum phosphate reabsorption per glomerular filtration rate (TmP/GFR)] from entry through week 96 in those initiating Tenofovir ( n = 134) versus abacavir ( n = 135) with efavirenz or atazanavir/ritonavir in A5224s. We also correlated changes in BMD with Tenofovir AUC measured between weeks 4 and 24. Results: Changes in TRP and TmP/GFR through week 96 between the Tenofovir and abacavir arms were not significantly different (both P >/= 0.70) and did not differ with use of efavirenz versus atazanavir/ritonavir. There were no significant correlations between changes in either TRP or TmP/GFR and with either hip or spine BMD in the Tenofovir arms. Tenofovir AUC was significantly correlated with changes in hip BMD, but not spine BMD, at week 24 ( r = -0.22, P = 0.028) and week 48 ( r = -0.26, P = 0.010), but not at week 96 ( r = -0.14, P = 0.18). Conclusions: Changes in phosphaturia were not different between the Tenofovir and abacavir arms in A5224s. Changes in hip and spine BMD with Tenofovir were not related to changes in phosphaturia. However, Tenofovir exposure was weakly associated with changes in hip BMD through week 48.
Selective inhibition of HIV-1 reverse transcriptase by an antiviral inhibitor, (R)-9-(2-Phosphonylmethoxypropyl)adenine.[Pubmed:9765248]
J Biol Chem. 1998 Oct 16;273(42):27250-8.
(R)-9-(2-Phosphonylmethoxypropyl)adenine (PMPA) is an acyclic nucleoside phosphonate that has been shown to be effective in the treatment of AIDS although it has a shorter separation between the adenine and phosphorus than dideoxy-AMP and dAMP. By using pre-steady state kinetic methods, we examined the incorporation of the diphosphate of PMPA, 2',3'-dideoxyadenosine 5'-triphosphate (ddATP), and dATP catalyzed by wild-type human immunodeficiency virus type 1 (HIV-1) reverse transcriptase, an exonuclease-deficient T7 DNA polymerase (T7 exo-), and wild-type rat DNA polymerase beta in order to evaluate the selectivity of PMPA as an antiviral inhibitor. With a DNA/DNA or DNA/RNA 22/43-mer duplex, the diphosphate of PMPA (PMPApp) is as effective as ddATP in reactions catalyzed by HIV-1 reverse transcriptase in that both analogs have similar substrate specificity constants (kp/Kd) which are only 5-fold lower than dATP. In contrast, PMPApp is a much weaker inhibitor of the reaction catalyzed by T7 exo- (with the DNA/DNA 22/43-mer duplex) in that PMPApp has a 5 x 10(-4)-fold lower kp/Kd than ddATP and dATP. The lower kp/Kd of PMPApp is due to a 1000-2000-fold lower incorporation rate (kp) and a 35-45-fold lower binding constant (Kd). Similarly, PMPApp is 800-fold less inhibitory toward polymerase beta with the DNA/DNA 22/43-mer duplex, whereas in studies with a single nucleotide gapped DNA (22-20/43-mer) PMPApp is 13-fold less inhibitory than ddATP. Although parallel studies will need to be performed using appropriate human polymerases, these results begin to define the mechanistic basis for the reported lower toxicity of PMPA in the treatment of AIDS.
Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine.[Pubmed:7502044]
Science. 1995 Nov 17;270(5239):1197-9.
The efficacy of pre- and postexposure treatment with the antiviral compound (R)-9-(2-phosphonylmethoxypropyl)adenine (PMPA) was tested against simian immunodeficiency virus (SIV) in macaques as a model for human immunodeficiency virus (HIV). PMPA was administered subcutaneously once daily beginning either 48 hours before, 4 hours after, or 24 hours after virus inoculation. Treatment continued for 4 weeks and the virologic, immunologic, and clinical status of the macaques was monitored for up to 56 weeks. PMPA prevented SIV infection in all macaques without toxicity, whereas all control macaques became infected. These results suggest a potential role for PMPA prophylaxis against early HIV infection in cases of known exposure.


