TT 232sst1/sst4 somatostatin receptor agonist CAS# 147159-51-1 |
- Vatalanib (PTK787) 2HCl
Catalog No.:BCC1111
CAS No.:212141-51-0
- Ki8751
Catalog No.:BCC1116
CAS No.:228559-41-9
- Cediranib (AZD217)
Catalog No.:BCC1121
CAS No.:288383-20-0
- Lenvatinib (E7080)
Catalog No.:BCC1172
CAS No.:417716-92-8
- Tivozanib (AV-951)
Catalog No.:BCC1179
CAS No.:475108-18-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 147159-51-1 | SDF | Download SDF |
| PubChem ID | 164469 | Appearance | Powder |
| Formula | C45H58N10O9S2 | M.Wt | 947.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
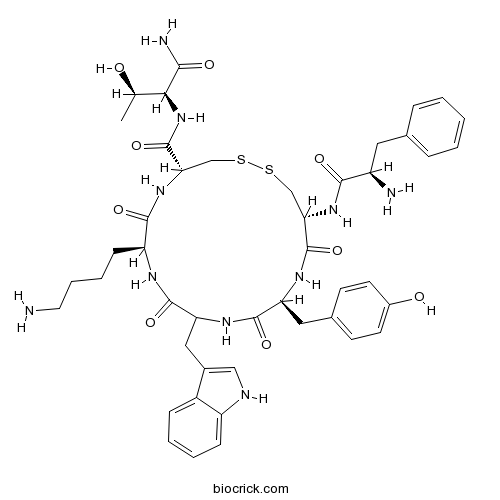
| Sequence | fCYwKCT (Modifications: Disulfide bridge between 2 - 6; Thr-7 = C-terminal amide) | ||
| Chemical Name | (4R,7S,13S,16R)-7-(4-aminobutyl)-N-[(2S,3R)-1-amino-3-hydroxy-1-oxobutan-2-yl]-16-[[(2R)-2-amino-3-phenylpropanoyl]amino]-13-[(4-hydroxyphenyl)methyl]-10-(1H-indol-3-ylmethyl)-6,9,12,15-tetraoxo-1,2-dithia-5,8,11,14-tetrazacycloheptadecane-4-carboxamide | ||
| SMILES | CC(C(C(=O)N)NC(=O)C1CSSCC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CCCCN)CC2=CNC3=CC=CC=C32)CC4=CC=C(C=C4)O)NC(=O)C(CC5=CC=CC=C5)N)O | ||
| Standard InChIKey | SNAJPQVDGYDQSW-QKVIHVPISA-N | ||
| Standard InChI | InChI=1S/C45H58N10O9S2/c1-25(56)38(39(48)58)55-45(64)37-24-66-65-23-36(53-40(59)31(47)19-26-9-3-2-4-10-26)44(63)51-34(20-27-14-16-29(57)17-15-27)42(61)52-35(21-28-22-49-32-12-6-5-11-30(28)32)43(62)50-33(41(60)54-37)13-7-8-18-46/h2-6,9-12,14-17,22,25,31,33-38,49,56-57H,7-8,13,18-21,23-24,46-47H2,1H3,(H2,48,58)(H,50,62)(H,51,63)(H,52,61)(H,53,59)(H,54,60)(H,55,64)/t25-,31-,33+,34+,35?,36+,37+,38+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Peptide agonist for sst1/sst4 somatostatin receptors. Somatostatin analog; inhibits tyrosine kinase activity in human colon tumor cell lines. Demonstrates an antiproliferative effect both in vitro and in vivo and induces apoptosis in a pancreatic tumor cell line. Does not inhibit growth hormone release or secretion. |

TT 232 Dilution Calculator

TT 232 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Chloro-L-phenylalanine Hydrochloride
Catalog No.:BCC2638
CAS No.:123053-23-6
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- 4-(4-Fluorophenyl)-6-isopropyl-2-[(N-methyl-N-methylsulfonyl)amino]pyrimidinyl-5-yl-formyl
Catalog No.:BCC8651
CAS No.:147118-37-4
- Methyl (3R)-3-(tert-butyldimethylsilyloxy)-5-oxo-6-triphenylphosphoranylidenehexanoate
Catalog No.:BCC9031
CAS No.:147118-35-2
- Maropitant
Catalog No.:BCC1728
CAS No.:147116-67-4
- Rosuvastatin Calcium
Catalog No.:BCC3853
CAS No.:147098-20-2
- 5,6-Dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-4-one
Catalog No.:BCC8722
CAS No.:147086-79-1
- Alcaftadine
Catalog No.:BCC5260
CAS No.:147084-10-4
- Trovafloxacin mesylate
Catalog No.:BCC3931
CAS No.:147059-75-4
- Rocaglaol
Catalog No.:BCN1653
CAS No.:147059-46-9
- Cyclo(Phe-Pro)
Catalog No.:BCN2416
CAS No.:14705-60-3
- MK591
Catalog No.:BCC1766
CAS No.:147030-01-1
- N-6-Methyl-7,7-dioxo-2-sulfamoyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-yl]acetamide
Catalog No.:BCC9077
CAS No.:147200-03-1
- Arecaidine propargyl ester tosylate
Catalog No.:BCC6628
CAS No.:147202-94-6
- Delavirdine mesylate
Catalog No.:BCC4069
CAS No.:147221-93-0
- glatiramer acetate
Catalog No.:BCC5642
CAS No.:147245-92-9
- 7ACC2
Catalog No.:BCC5554
CAS No.:1472624-85-3
- KRCA 0008
Catalog No.:BCC8007
CAS No.:1472795-20-2
- Antibiotic PF 1052
Catalog No.:BCN1828
CAS No.:147317-15-5
- Cylindramide
Catalog No.:BCN1832
CAS No.:147362-39-8
- MKT 077
Catalog No.:BCC6241
CAS No.:147366-41-4
- Ligupurpuroside A
Catalog No.:BCC8198
CAS No.:147396-01-8
- Ligupurpuroside B
Catalog No.:BCC8199
CAS No.:147396-02-9
- Azilsartan
Catalog No.:BCC5014
CAS No.:147403-03-0
Evaluation of the antitumor efficacy of the somatostatin structural derivative TT-232 on different tumor models.[Pubmed:17094470]
Anticancer Res. 2006 Sep-Oct;26(5A):3477-83.
The antitumor effects of the somatostatin structural derivative TT-232 in different rodent and xenograft tumor models are summarized in this report. TT-232 had previously been shown to inhibit the proliferation of a large number of cancer cell lines in vitro and reduce the size of different tumors in animal models in vivo. The effects of TT-232 by different routes of administration and treatment schedules were studied in various types of rodent and human xenograft tumor models. In the rodent tumor models S-180 sarcoma and P-388 lymphoid leukemia tumor the infusion treatment resulted in 76%-100% tumor growth inhibition and in 20%-60% of the mice being long-term and tumor-free survivors. In the aggressive C-26 colon carcinoma and MXT breast carcinoma, the TT-232 treatments resulted in 71%-75% tumor growth inhibition and an approximately 50% increased survival time. The tumor growth inhibitory effect of TT-232 on human tumor xenografts proved to be significant, resulting in 30%-80% decrease in tumor volume and in 20%-40% tumor-free animals. This antitumor efficacy of TT-232 was seen in almost all the tumors investigated. In our study, the route of infusion was shown to increase drug efficacy relative to conventional delivery methods. Our results suggested that TT-232 is an effective and promising antitumor agent.
Antitumor activity of the somatostatin structural derivative (TT-232), against mouse and human melanoma tumor models.[Pubmed:18225564]
Anticancer Res. 2007 Nov-Dec;27(6B):4015-9.
BACKGROUND: The somatostatin structural deivative, TT-232, has a special 5'-residue ring structure (D-Phe-Cys-Tyr-D-Trp-Lys-Cys-Thr-NH2) and very different characteristics from the known growth hormone (GH) active somatostatin analogs. TT-232 inhibited tyrosine kinase activity of tumor cell lines and this inhibition correlated well with the inhibition of cell proliferation of a large number of cancer cell lines in vitro and reduces the size of different tumors in animal models in vivo. The antitumor efficacy of TT-232 has been found to be associated with the induction of apoptosis in tumor cells, resulting in highly selective elimination of tumor tissue. TT-232 was found to be devoid of GH release inhibitory activity but to possess strong antitumor effects. It binds with a high affinity to SSTR1 and SSTR4. This compound was also found to inhibit inflammation in a number of experimental models. MATERIALS AND METHODS: The study compared the antitumor effect of TT-232 in various long-term administration routes: an intermittent (injection) versus continuous (infusion) treatment via subcutaneously inserted 2002 type Alzet osmotic minipumps in two different tumor models (B-16 rodent melanoma and HT-18 human lymphoid melanoma). Treatment with TT-232 started after disease development. The antitumor efficacy of TT-232 was evaluated on the basis of tumor growth inhibition and survival time. RESULTS: In the case of B-16 rodent melanoma, the TT-232 treatments resulted in 35%-39% (injection) and 47%-63% (infusion) tumor growth inhibition, and the infusion treatment an approximately 61% increase in survival time. The tumor growth inhibitory effect of TT-232 on HT-18 lymphoid melanoma tumor proved to be significant, resulting in 41%-63% (injection) and 69%-79% (infusion) decreases in tumor volume and in a 25%-30% increase in survival time (infusion treatments). CONCLUSION: The results indicate that TT-232 could be a potentially useful therapeutic agent if these data are translated into clinical practice.
A comparison of the tumor growth inhibitory effect of intermittent and continuous administration of the somatostatin structural derivative TT-232 in various human tumor models.[Pubmed:16886628]
Anticancer Res. 2006 Jul-Aug;26(4B):3011-5.
The tumor growth inhibitory efficacy of the somatostatin structural derivative TT-232 was studied using different routes of administration and treatment schedules in various human tumor models. TT-232, containing a five-residue ring structure, has a strong antitumor activity both in vitro and in vivo. The antineoplastic activity of TT-232 has been found to be associated with the induction of programmed cell death in tumor cells, resulting in highly-selective elimination of the neoplastic tissue. The study compared the antitumor efficacy of TT-232 in various long-term administration routes; the intermittent (injection) versus continuous (infusion) treatment via subcutaneously-inserted Alzet osmotic minipumps in different human tumor models: T-47/D human breast carcinoma and A-431 human epidermoid carcinoma. Treatment with TT-232 started after disease development. The antitumor activity of TT-232 was evaluated on the basis of the tumor growth inhibition. In the case of T-47/D human breast carcinoma, the intermittent treatment resulted in 23%-26% and the infusion treatment resulted in 48%-53% tumor growth inhibition. The tumor growth inhibitory effect of TT-232 on A-431 human epidermoid carcinoma tumor resulted in 35%-43% (intermittent treatment) and 70%-74% (continuous treatment) decreases in tumor volume. This antitumor efficacy of TT-232 was observed in the two human tumors investigated. In this study, the route of infusion was shown to increase drug efficacy relative to conventional delivery methods. The results suggest that TT-232 is an effective and promising antitumor agent.
Continuous administration of the somatostatin structural derivative /TT-232/ by subcutaneously implanted osmotic pump improves the efficacy and potency of antitumor therapy in different mouse and human tumor models.[Pubmed:19035308]
Anticancer Res. 2008 Sep-Oct;28(5A):2769-74.
BACKGROUND: The somatostatin structural derivative, TT-232, has a special 5-residue ring structure (D-Phe-Cys-Tyr-D-Trp-Lys-Cys-Thr-NH2) and very different characteristics from the known growth hormone (GH) active somatostatin analogs. This somatostatin structural derivative has no GH release inhibitory or antisecretory activity and does not bind to rat pituitary or the cortex, where all the known somatostatin receptor subtypes are expressed. TT-232 had previously been shown to inhibit the proliferation of a large number of cancer cell lines in vitro and reduce the size of different tumors in animal models in vivo. MATERIALS AND METHODS: The therapeutic efficacy of TT-232 was evaluated in different long-term administration routes: the traditional injection (i.p. or s.c.) versus infusion treatment via s.c.- or i.v.-inserted Alzet osmotic minipump, and on different types of transplantable rodent (S-180 sarcoma, P-388sc lymphoid leukemia, Colon-26 adenocarcinoma, MXT breast carcinoma, B-16 melanoma) and human tumor models (HT-18 lymphoid melanoma, T-47/D breast carcinoma, A-431 epidermoid carcinoma). On the basis of our previous experiments the optimum injected dose of TT-232 was found to be 15 microg/kg twice a day. This dose is equivalent to 0.6 dg/day by infusion therapy. RESULTS: In our experiments, the best results were achieved when TT-232 was applied as an infused treatment. In the S-180 sarcoma and P-388sc lymphoid leukemia rodent tumor models the infusion treatment with TT-232 resulted in 61%-100% tumor growth inhibition and in 20%-60% of the mice being long-term and tumor-free survivors. In the aggressive Colon-26 adenocarcinoma and MXT breast carcinoma models, the infusion treatment resulted in 52%-75% tumor growth inhibition. In the B-16 melanoma model, the infusion treatments resulted in 47% -63% growth inhibition. The tumor growth inhibitory effect of infusion treatment with TT-232 on HT-18 human lymphoid melanoma tumor proved to be significant, resulting in 69%-79% decreases in tumor volume. In the T-47/D human breast carcinoma, the infusion treatment resulted in 48%-53% tumor growth inhibition. The tumor growth inhibitory effect of infusion treatment on A-431 human epidermoid carcinoma tumor resulted in 70%-74% decreases in tumor volume. CONCLUSION: The antitumor efficacy of TT-232 was seen in almost all the tumors investigated. In our study, the route of infusion was shown to increase drug efficacy relative to conventional delivery methods (injection). The results obtained from this study suggest that TT-232 is a promising new antitumor agent in cancer chemotherapy and a good candidate for delivery by continuous (infusion) therapy.
Inhibitory effects of synthetic somatostatin receptor subtype 4 agonists on acute and chronic airway inflammation and hyperreactivity in the mouse.[Pubmed:17961545]
Eur J Pharmacol. 2008 Jan 14;578(2-3):313-22.
Somatostatin released from activated capsaicin-sensitive afferents of the lung inhibits inflammation and related bronchial hyperreactivity presumably via somatostatin 4 receptors (sst(4)). The aim of this study was to examine the effects of TT-232, a heptapeptide sst(4)/sst(1) receptor agonist and J-2156, a high affinity sst(4) receptor-selective peptidomimetic agonist in airway inflammation models. Acute pneumonitis was evoked by intranasal lipopolysaccharide 24 h before measurement. Chronic inflammation was induced by ovalbumin inhalation on days 28, 29 and 30 after i.p. sensitization on days 1 and 14. Semiquantitative histopathological scoring was based on perivascular/peribronchial oedema, neutrophil/macrophage infiltration, goblet cell hyperplasia in the acute model and eosinophil infiltration, mucosal oedema, mucus production and epithelial cell damage in chronic inflammation. Myeloperoxidase activity of the lung was measured spectrophotometrically to quantify granulocyte accumulation and the broncoalveolar lavage fluid was analysed by flow cytometry. Carbachol-induced bronchoconstriction was assessed by unrestrained whole body plethysmography and its calculated indicator, enhanced pause (Penh) was determined. TT-232 and J-2156 induced similar inhibition on granulocyte recruitment and histopathological changes in both models, although macrophage infiltration in LPS-induced inflammation was unaltered by either compounds. Both agonists diminished inflammatory airway hyperresponsiveness. Since their single administration after the development of the inflammatory reactions also inhibited carbachol-induced bronchoconstriction, somatostatin sst(4) receptor activation on bronchial smooth muscle cells is likely to be involved in their anti-hyperreactivity effect. These results suggest that stable, somatostatin sst(4) receptor-selective agonists could be potential candidates for the development of a completely novel group of anti-inflammatory drugs for the treatment of airway inflammation and hyperresponsiveness.
A tumor-selective somatostatin analog (TT-232) with strong in vitro and in vivo antitumor activity.[Pubmed:8901613]
Proc Natl Acad Sci U S A. 1996 Oct 29;93(22):12513-8.
We report a series of new in vitro and in vivo data proving the selective antitumor activity of our somatostatin structural derivative, TT-232. In vitro, it inhibited the proliferation of 20 different human tumor cell lines in the range of 50-95% and induced a very strong apoptosis. In vivo TT-232 was effective on transplanted animal tumors (Colon 26, B16 melanoma, and S180 sarcoma) and on human tumor xenografts. Treatment of MDA-MB-231 human breast cancer xenografted in mice with low submaximal doses of TT-232 [0.25 and 0.5 mg/kg of body weight (b.w.)] caused an average 80% decrease in the tumor volume resulting in 30% tumor-free animals surviving for longer than 200 days. Treatment of prostate tumor (PC-3) xenografted animals with 20 mg/kg of b.w. of TT-232 for 3 weeks resulted in 60% decrease in tumor volume and 100% survival even after 60 days, while 80% of nontreated animals perished. We have demonstrated that TT-232 did not bind to the membrane preparation of rat pituitary and cortex and had no antisecretory activity. TT-232 was not toxic at a dose of 120 mg/kg of b.w. in mice. Long-term incubation (24 h) of tumor cells with TT-232 caused significant inhibition of tyrosine kinases in good correlation with the apoptosis-inducing effect. The level of p53 or KU86 did not change following TT-232 treatment, suggesting a p53-independent apoptotic effect. Preincubation of human breast cancer cells (MDA-MB-453) with TT-232 for 2 h decreased the growth factor receptor autophosphorylation. All of these data suggest that TT-232 is a promising and selective antitumor agent.


