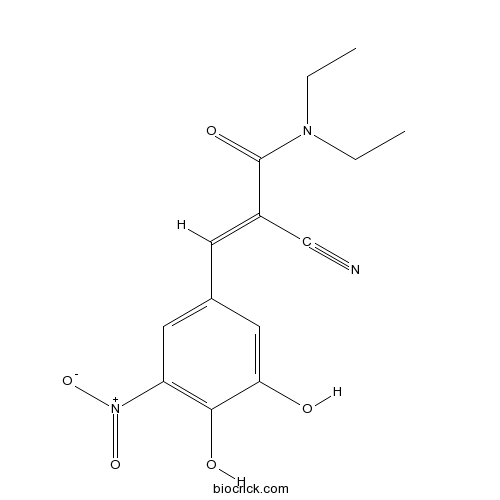
EntacaponeCOMT inhibitor CAS# 130929-57-6 |
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- CCT137690
Catalog No.:BCC2188
CAS No.:1095382-05-0
- CYC116
Catalog No.:BCC2181
CAS No.:693228-63-6
- MLN8054
Catalog No.:BCC2170
CAS No.:869363-13-3
- TAK-901
Catalog No.:BCC2180
CAS No.:934541-31-8
- PF-03814735
Catalog No.:BCC2184
CAS No.:942487-16-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 130929-57-6 | SDF | Download SDF |
| PubChem ID | 5281081 | Appearance | Powder |
| Formula | C14H15N3O5 | M.Wt | 305.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 33.33 mg/mL (109.17 mM; Need ultrasonic) H2O : 2 mg/mL (6.55 mM; ultrasonic and adjust pH to 10 with NaOH) | ||
| Chemical Name | (E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethylprop-2-enamide | ||
| SMILES | CCN(CC)C(=O)C(=CC1=CC(=C(C(=C1)O)O)[N+](=O)[O-])C#N | ||
| Standard InChIKey | JRURYQJSLYLRLN-BJMVGYQFSA-N | ||
| Standard InChI | InChI=1S/C14H15N3O5/c1-3-16(4-2)14(20)10(8-15)5-9-6-11(17(21)22)13(19)12(18)7-9/h5-7,18-19H,3-4H2,1-2H3/b10-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent catechol O-methyltransferase (COMT) inhibitor (IC50 values are 14.3, 20.1 and 73.3 nM for rat liver soluble COMT, total COMT and membrane-bound COMT respectively). Increases bioavailability of levodopa when given as an adjunct therapy for Parkinson's disease. Inhibits α-synuclein aggregation in an in vitro assay; blocks α-synuclein-induced cell death in PC-12 cells. |

Entacapone Dilution Calculator

Entacapone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2756 mL | 16.3779 mL | 32.7557 mL | 65.5115 mL | 81.8894 mL |
| 5 mM | 0.6551 mL | 3.2756 mL | 6.5511 mL | 13.1023 mL | 16.3779 mL |
| 10 mM | 0.3276 mL | 1.6378 mL | 3.2756 mL | 6.5511 mL | 8.1889 mL |
| 50 mM | 0.0655 mL | 0.3276 mL | 0.6551 mL | 1.3102 mL | 1.6378 mL |
| 100 mM | 0.0328 mL | 0.1638 mL | 0.3276 mL | 0.6551 mL | 0.8189 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Entacapone is a second-generation catechol-O-methyltransferase (COMT) inhibitor that potently inhibits rat liver total COMT with the half maximal inhibition concentration IC50 and inhibition constant Ki values of 20.1 nM and 10.7 nM respectively. The IC50 values of entacapone against rate liver soluble COMT (S-COMT) and rat liver membrane-bound COMT (MB-COMT) are 14.3 nM and 73.3 nM respectively [1].
Entacapone has succbe used as an adjunct to standard levodopa-dopa decarboxylase inhibitor therapy in patients with Parkinson’s disease (PD), for its abilities to increase the bioavailability of levodopa by inhibiting the generation of 3-O-methyldopa and to prolong the duration and clinical benefit of levodopa [1].
Reference
References:
[1] Forsberg M, Lehtonen M, Heikkinen M, Savolainen J, Järvinen T, Männistö PT. Pharmacokinetics and pharmacodynamics of entacapone and tolcapone after acute and repeated administration: a comparative study in the rat. J Pharmacol Exp Ther. 2003 Feb;304(2):498-506.
- 7-O-Acetylneocaesalpin N
Catalog No.:BCN7332
CAS No.:1309079-08-0
- N4-Benzoylcytidine
Catalog No.:BCC9072
CAS No.:13089-48-0
- Fmoc-Val-OSu
Catalog No.:BCC3572
CAS No.:130878-68-1
- 1beta,10beta-Epoxydehydroleucodin
Catalog No.:BCN7331
CAS No.:130858-00-3
- ent-kaurane-3,16,17-triol
Catalog No.:BCN6164
CAS No.:130855-22-0
- Decursinol angelate
Catalog No.:BCC9222
CAS No.:130848-06-5
- Scoparinol
Catalog No.:BCN6163
CAS No.:130838-00-5
- 7-Oxohinokinin
Catalog No.:BCN6162
CAS No.:130837-92-2
- Pseudolarifuroic acid
Catalog No.:BCN8048
CAS No.:130825-79-5
- Sipatrigine
Catalog No.:BCC7847
CAS No.:130800-90-7
- MDL-29951
Catalog No.:BCC4059
CAS No.:130798-51-5
- 6-O-benzoylgomisin O
Catalog No.:BCN3092
CAS No.:130783-32-3
- CX-4945 sodium salt
Catalog No.:BCC5586
CAS No.:1309357-15-0
- Cerberic acid B
Catalog No.:BCN4715
CAS No.:1309362-77-3
- GPR40 Activator 1
Catalog No.:BCC4125
CAS No.:1309435-60-6
- K145
Catalog No.:BCC4305
CAS No.:1309444-75-4
- Wittifuran X
Catalog No.:BCN4794
CAS No.:1309478-07-6
- Marumoside A
Catalog No.:BCN7702
CAS No.:1309604-34-9
- H 89 2HCl
Catalog No.:BCC4997
CAS No.:130964-39-5
- (±)-CPSI 1306
Catalog No.:BCC6161
CAS No.:1309793-47-2
- Sephin1
Catalog No.:BCC3980
CAS No.:13098-73-2
- MRS 4062 triethylammonium salt
Catalog No.:BCC6134
CAS No.:1309871-50-8
- 15-Methoxymkapwanin
Catalog No.:BCN6498
CAS No.:1309920-99-7
- VU 0360172 hydrochloride
Catalog No.:BCC6141
CAS No.:1309976-62-2
Levodopa-entacapone-carbidopa intestinal gel in Parkinson's disease: A randomized crossover study.[Pubmed:27987231]
Mov Disord. 2017 Feb;32(2):283-286.
BACKGROUND: The addition of oral Entacapone to levodopa-carbidopa intestinal gel treatment leads to less conversion of levodopa to 3-O-methyldopa, thereby increasing levodopa plasma concentration. The objective of this study was to compare systemic levodopa exposure of the newly developed levodopa-Entacapone-carbidopa intestinal gel after a 20% dose reduction with levodopa exposure after the usual levodopa-carbidopa intestinal gel dose in a randomized crossover trial in advanced Parkinson's disease patients. METHODS: In this 48-hour study, 11 patients treated with levodopa-carbidopa intestinal gel were randomized to a treatment sequence. Blood samples were drawn at prespecified times, and patient motor function was assessed according to the treatment response scale. RESULTS: Systemic exposure of levodopa did not differ significantly between treatments (ratio, 1.10 [95% confidence interval, 0.951-1.17]). Treatment response scale scores did not significantly differ between treatments (P = 0.84). CONCLUSIONS: Levodopa-Entacapone-carbidopa intestinal gel allowed a lower amount of levodopa administration and was well tolerated. Long-term studies are needed to confirm the results. (c) 2016 International Parkinson and Movement Disorder Society.
Micelle-enhanced spectrofluorimetric method for quantification of entacapone in tablets and human plasma.[Pubmed:27917581]
Luminescence. 2017 Aug;32(5):713-722.
In this paper, a simple and highly sensitive spectrofluorimetric method was developed and validated for the determination of Entacapone (ETC). The proposed method is based on forming a highly fluorescent product through the reduction of ETC with Zn/HCl. The produced fluorophore exhibits strong fluorescence at lambdaem 345 nm after excitation at lambdaex 240 nm. The use of fluorescence enhancers such as Tween-80 and carboxy methyl cellulose (CMC) greatly enhanced the fluorescence of the produced fluorophore by 150% and 200%, respectively. Calibration curves showed good linear regression (r2 > 0.9998) within test ranges of 0.05-2.0 and 0.02-1.80 mug mL(-1) with lower detection limits of 1.27 x 10(-2) and 4.8 x 10(-3) mug mL(-1) and lower quantification limits of 4.21 x 10(-2) and 1.61 x 10(-2) mug mL(-1) upon using Tween-80 and or CMC, respectively. The method was successfully applied to the analysis of ETC in its pharmaceutical formulations (either alone or in presence of other co-formulated drugs). The results were in good agreement with those obtained using the official method. The methods were further extended to determine the drug in human plasma samples, and to study the pharmacokinetics of ETC. The paper is the first report on the spectrofluorimetric determination of Entacapone.
Homocysteine Levels in Parkinson's Disease: Is Entacapone Effective?[Pubmed:27493964]
Biomed Res Int. 2016;2016:7563705.
Plasma homocysteine (Hcy) levels may increase in levodopa-treated patients with Parkinson's disease (PD) as a consequence of levodopa methylation via catechol-O-methyltransferase (COMT). Results from previous studies that assessed the effect of COMT inhibitors on levodopa-induced hyperhomocysteinemia are conflicting. We aimed to evaluate the effects of levodopa and Entacapone on plasma Hcy levels. A hundred PD patients were enrolled to the study and divided into three treatment groups (group I: levodopa and/or dopamine agonists; group II: levodopa, Entacapone, and/or a dopamine agonist; and group III: dopamine agonist alone). We measured the serum B12, folic acid, and Hcy levels in all patients. There were no statistically significant differences between groups in terms of modified Hoehn and Yahr stages, Unified Parkinson's Disease Rating Scale II/III, Standardized Mini-Mental Test scores, and serum vitamin B12 and folic acid levels. Plasma median Hcy levels were found above the normal laboratory values in groups I and II, but they were normal in group III. However, there was no statistically significant difference in plasma Hcy levels between groups. Our results showed that levodopa treatment may cause a slight increase in the Hcy levels in PD compared with dopamine agonists and that COMT inhibitors may not have a significant effect on preventing hyperhomocysteinemia.
Investigation of different spectrophotometric and chemometric methods for determination of entacapone, levodopa and carbidopa in ternary mixture.[Pubmed:27541796]
Spectrochim Acta A Mol Biomol Spectrosc. 2017 Jan 15;171:236-245.
New, simple, accurate and sensitive UV spectrophotometric and chemometric methods have been developed and validated for determination of Entacapone (ENT), Levodopa (LD) and Carbidopa (CD) in ternary mixture. Method A is a derivative ratio spectra zero-crossing spectrophotometric method which allows the determination of ENT in the presence of both LD and CD by measuring the peak amplitude at 249.9nm in the range of 1-20mugmL(-1). Method B is a double divisor-first derivative of ratio spectra method, used for determination of ENT, LD and CD at 245, 239 and 293nm, respectively. Method C is a mean centering of ratio spectra which allows their determination at 241, 241.6 and 257.1nm, respectively. Methods B and C could successfully determine the studied drugs in concentration ranges of 1-20mugmL(-1) for ENT and 10-90mugmL(-1) for both LD and CD. Methods D and E are principal component regression and partial least-squares, respectively, used for the simultaneous determination of the studied drugs by using seventeen mixtures as calibration set and eight mixtures as validation set. The developed methods have the advantage of simultaneous determination of the cited components without any pre-treatment. All the results were statistically compared with the reported methods, where no significant difference was observed. The developed methods were satisfactorily applied to the analysis of the investigated drugs in their pure form and in pharmaceutical dosage forms.
Entacapone and tolcapone, two catechol O-methyltransferase inhibitors, block fibril formation of alpha-synuclein and beta-amyloid and protect against amyloid-induced toxicity.[Pubmed:20150427]
J Biol Chem. 2010 May 14;285(20):14941-54.
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease (AD). There is considerable consensus that the increased production and/or aggregation of alpha-synuclein (alpha-syn) plays a central role in the pathogenesis of PD and related synucleinopathies. Current therapeutic strategies for treating PD offer mainly transient symptomatic relief and aim at the restitution of dopamine levels to counterbalance the loss of dopaminergic neurons. Therefore, the identification and development of drug-like molecules that block alpha-synuclein aggregation and prevent the loss of dopaminergic neurons are desperately needed to treat or slow the progression of PD. Here, we show that Entacapone and tolcapone are potent inhibitors of alpha-syn and beta-amyloid (Abeta) oligomerization and fibrillogenesis, and they also protect against extracellular toxicity induced by the aggregation of both proteins. Comparison of the anti-aggregation properties of Entacapone and tolcapone with the effect of five other catechol-containing compounds, dopamine, pyrogallol, gallic acid, caffeic acid, and quercetin on the oligomerization and fibrillization of alpha-syn and Abeta, demonstrate that the catechol moiety is essential for the anti-amyloidogenic activity. Our findings present the first characterization of the anti-amyloidogenic properties of tolcapone and Entacapone against both alpha-synuclein and Abeta42 and highlight the potential of this class of nitro-catechol compounds as anti-amyloidogenic agents. Their inhibitory properties, mode of action, and structural properties suggest that they constitute promising lead compounds for further optimization.
Pharmacokinetics and pharmacodynamics of entacapone and tolcapone after acute and repeated administration: a comparative study in the rat.[Pubmed:12538800]
J Pharmacol Exp Ther. 2003 Feb;304(2):498-506.
Two catechol-O-methyltransferase (COMT) inhibitors, Entacapone and tolcapone, were compared in the rat to elucidate the actual differences between their pharmacokinetics and pharmacodynamics after single and repeated administration. Their inhibitory potencies were also compared in vitro. After intravenous administration (3 mg/kg), the elimination half-life (t(1/2 beta)) of Entacapone (0.8 h) was clearly shorter than that of tolcapone (2.9 h). The striatum/serum ratio of tolcapone was 3-fold higher than that of Entacapone. After a single oral dose (10 mg/kg), both Entacapone and tolcapone produced an equal maximal degree of COMT inhibition in peripheral tissues, but tolcapone inhibited striatal COMT more effectively than did Entacapone. After the 7-day treatment (10 mg/kg twice daily), COMT activity had recovered to a level of 67 to 101% of control within 8 h after the last dose of Entacapone. In tolcapone-treated animals, there was still extensive COMT inhibition present in peripheral tissues, and the degree of inhibition was higher than that attained after a single dose. The pharmacokinetic-pharmacodynamic modeling revealed that a plateau of COMT inhibition near the maximal attainable inhibition was reached already by plasma concentrations below 2000 ng/ml, both with Entacapone and tolcapone. Entacapone and tolcapone inhibited equally rat liver COMT in vitro with K(i) values of 10.7 and 10.0 nM, respectively. In conclusion, tolcapone has a longer duration of action and a better brain penetration than Entacapone. The results also suggest that peripheral COMT is inhibited continuously when tolcapone is dosed at 12-h intervals, but this was not seen with Entacapone.
Effect of entacapone, a peripherally acting catechol-O-methyltransferase inhibitor, on the motor response to acute treatment with levodopa in patients with Parkinson's disease.[Pubmed:8126502]
J Neurol Neurosurg Psychiatry. 1994 Feb;57(2):186-9.
Catechol-O-methyltransferase (COMT) inhibitors may be useful in the treatment of Parkinson's disease by improving the bioavailability of levodopa and by prolonging its effects. Entacapone (OR-611), a novel COMT inhibitor, which does not cross the blood brain barrier, was assessed in 12 patients with Parkinson's disease and motor fluctuations in a randomised, double-blind, cross-over, single dose study. The magnitude and duration of the therapeutic response to a single dose of 200 mg levodopa/50 mg carbidopa was evaluated after concomitant placebo, or 200 or 800 mg Entacapone. A significant increase in the duration of the motor response to levodopa was seen when 200 mg Entacapone was given with levodopa/carbidopa. Plasma levodopa concentrations were increased with both doses of the COMT inhibitor. The latency to onset of motor response did not differ significantly between active drug and placebo. Entacapone may prove useful in prolonging the duration of the benefit obtained from individual doses of levodopa.


