MLN8237 (Alisertib)Aurora A Kinase inhibitor, Potent and selective CAS# 1028486-01-2 |
- CCT137690
Catalog No.:BCC2188
CAS No.:1095382-05-0
- SNS-314 Mesylate
Catalog No.:BCC2177
CAS No.:1146618-41-8
- Aurora A Inhibitor I
Catalog No.:BCC2182
CAS No.:1158838-45-9
- MLN8054
Catalog No.:BCC2170
CAS No.:869363-13-3
- MK-8745
Catalog No.:BCC3994
CAS No.:885325-71-3
- ENMD-2076
Catalog No.:BCC2186
CAS No.:934353-76-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure
3D structure
| Cas No. | 1028486-01-2 | SDF | Download SDF |
| PubChem ID | 24771867 | Appearance | Powder |
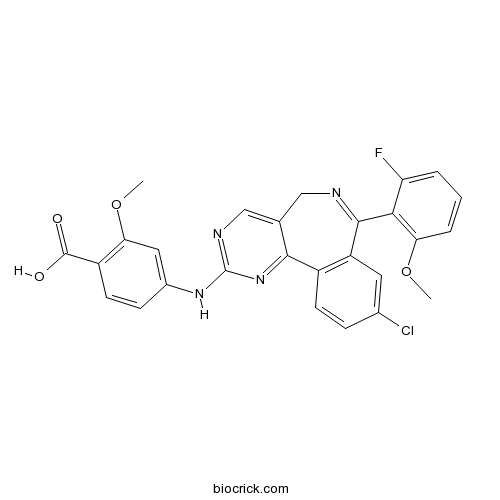
| Formula | C27H20ClFN4O4 | M.Wt | 518.92 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | MLN 8237 | ||
| Solubility | DMSO : 41.67 mg/mL (80.30 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 4-[[9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-pyrimido[5,4-d][2]benzazepin-2-yl]amino]-2-methoxybenzoic acid | ||
| SMILES | COC1=C(C(=CC=C1)F)C2=NCC3=CN=C(N=C3C4=C2C=C(C=C4)Cl)NC5=CC(=C(C=C5)C(=O)O)OC | ||
| Standard InChIKey | ZLHFILGSQDJULK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H20ClFN4O4/c1-36-21-5-3-4-20(29)23(21)25-19-10-15(28)6-8-17(19)24-14(12-30-25)13-31-27(33-24)32-16-7-9-18(26(34)35)22(11-16)37-2/h3-11,13H,12H2,1-2H3,(H,34,35)(H,31,32,33) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Alisertib (MLN8237) is a selective inhibitor of Aurora A with IC50 of 1.2 nM. It has >200-fold higher selectivity for Aurora A than Aurora B. | |||||
| Targets | Aurora A | |||||
| IC50 | 1.2 nM | |||||
| Cell experiment: [1] | |
| Cell lines | TIB-48 and CRL-2396 cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | >100 nM, 48 hours |
| Applications | TIB-48 and CRL-2396 cells were treated with MLN8237 at 10 nM, 50 nM, 100 nM, 500 nM and 1.0 μ M for 48 h. MLN8237 induced apoptosis at concentrations >100 nM, suggesting that induction of apoptosis is dose-dependent. These results were confirmed by demonstrating an increased level of cleaved PARP in treated TIB-48 and CRL-2396 cells. PARP cleavage was observed even at the concentration of MLN8237 as low as 50 nM. |
| Animal experiment: [2] | |
| Animal models | Female C.B-17 SCID mice injected with OVCAR-5-pWZL-Luc cells |
| Dosage form | Oral administration, 20 or 30 mg/kg, once daily (QD) or twice daily (BID) |
| Application | The mice (n=16/group) were randomly divided into five treatment groups: 1) vehicle, 2) 20 mg/kg alisertib, 3) 30 mg/kg alisertib, 4) 5 mg/kg paclitaxel and 5) 20 mg/kg alisertib + 5 mg/kg paclitaxel. Tumor growth was monitored by weekly BLI and the log-transformed total flux data showed significantly decreased tumor growth rates in mice treated with alisertib (20 or 30 mg/kg) compared to vehicle-treated mice. Treatment with 20 mg/kg and 30 mg/kg alisertib resulted in 51% and 49% TGI, respectively. |
| her notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Qi W, Spier C, Liu X, et al. Alisertib (MLN8237) an investigational agent suppresses Aurora A and B activity, inhibits proliferation, promotes endo-reduplication and induces apoptosis in T-NHL cell lines supporting its importance in PTCL treatment. Leukemia research, 2013, 37(4): 434-439. [2] Do T V, Xiao F, Bickel L E, et al. Aurora kinase A mediates epithelial ovarian cancer cell migration and adhesion. Oncogene, 2013, 33(5): 539-549. | |

MLN8237 (Alisertib) Dilution Calculator

MLN8237 (Alisertib) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9271 mL | 9.6354 mL | 19.2708 mL | 38.5416 mL | 48.177 mL |
| 5 mM | 0.3854 mL | 1.9271 mL | 3.8542 mL | 7.7083 mL | 9.6354 mL |
| 10 mM | 0.1927 mL | 0.9635 mL | 1.9271 mL | 3.8542 mL | 4.8177 mL |
| 50 mM | 0.0385 mL | 0.1927 mL | 0.3854 mL | 0.7708 mL | 0.9635 mL |
| 100 mM | 0.0193 mL | 0.0964 mL | 0.1927 mL | 0.3854 mL | 0.4818 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
The combination of vorinsostat and MLN8237, a Aurora A kinase inhibitor, has the potential to exhibit enhanced antitumor activity against pediatric tumor cell lines through disrupting the spindle assembly and the mitotic checkpoint.
Abstract
The safety, pharmacokinetics, pharmacodynamics and efficacy of MLN8237, an AAK inhibitor, has been assessed in adult patients with advanced solid tumors.
Abstract
Targeting Aurora kinase A is a potential treatment of bladder cancer.
Abstract
MLN8237 exhibited modest activity with the maximum tolerated dose of 50 mg BID on the ECT 7-day schedule and the terminal half-life of 19 hours in a phase I study evaluating two regimes of MLN8237 in patients with relapsed or refractory heme-lymphatic malignancies, in which MLN8237 ECT of 50 mg BID for 7 days in 21-day cycles is recommended.
Abstract
The safety, pharmacokinetics and pharmacodynamics of MLN8237, an AAK inhibitor, has been assessed in adult patients with advanced solid tumors.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
The orally bioavailable agent, MLN8237 (also known as alisertib), is a potent small-molecule inhibitor of Aurora A kinase (AAK) which is overexpressed in several types of tumor and associated with oncogenesis and tumor progression. It was developed from its predecessor, MLN8054, in order to minimize the benzodiazepine-like effects seen with MLN8054. The inhibitory effect of MLN8237 is ATP-competitive, reversible and AAK-specific with an inhibition constant (Ki) of 0.43 nmol/L. MLN8237 is being investigated to treat advanced malignancies, due to its both in vitro and in vivo activities against a broad range of tumor types.
Reference
E. Claire Dees, Roger B. Cohen, Margaret von Mehren, Thomas E. Stinchcombe, Hua Liu, Karthik Venkatakrishnan, Mark Manfredi, Howard Fingert, Howard A. Burris III, and Jeffrey R. Infante. Phase I study of aurora A kniase inhibitor MLN8237 in advanced solid tumors: safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin Cancer Res 2012; 18:4775-4784.
- Ganolactone B
Catalog No.:BCN2872
CAS No.:1028449-53-7
- Moracin P
Catalog No.:BCN3289
CAS No.:102841-46-3
- Mulberroside C
Catalog No.:BCN6344
CAS No.:102841-43-0
- Mulberroside A
Catalog No.:BCN6343
CAS No.:102841-42-9
- D-Pinitol
Catalog No.:BCN5842
CAS No.:10284-63-6
- BEZ235 Tosylate
Catalog No.:BCC1416
CAS No.:1028385-32-1
- VUF 10460
Catalog No.:BCC6285
CAS No.:1028327-66-3
- Dihydrocinchonamine
Catalog No.:BCN5841
CAS No.:10283-68-8
- RuBi-GABA
Catalog No.:BCC6012
CAS No.:1028141-88-9
- [D-p-Cl-Phe6,Leu17]-VIP
Catalog No.:BCC5968
CAS No.:102805-45-8
- GYKI 52466 dihydrochloride
Catalog No.:BCC7072
CAS No.:102771-26-6
- Levetiracetam
Catalog No.:BCC1056
CAS No.:102767-28-2
- Intermedine
Catalog No.:BCN1997
CAS No.:10285-06-0
- Lycopsamine
Catalog No.:BCN1999
CAS No.:10285-07-1
- 17-Hydroxy sprengerinin C
Catalog No.:BCN2755
CAS No.:1029017-75-1
- Pexidartinib (PLX3397)
Catalog No.:BCC6405
CAS No.:1029044-16-3
- MDL 73005EF hydrochloride
Catalog No.:BCC6636
CAS No.:102908-60-1
- Scutellaric acid
Catalog No.:BCN5843
CAS No.:102919-76-6
- INCB28060
Catalog No.:BCC3793
CAS No.:1029712-80-8
- Trelagliptin succinate
Catalog No.:BCC2015
CAS No.:1029877-94-8
- H-DL-Phg-OH
Catalog No.:BCC3317
CAS No.:103-01-5
- Monobenzone
Catalog No.:BCC3818
CAS No.:103-16-2
- Methyl cinnamate
Catalog No.:BCN5043
CAS No.:103-26-4
- Ethyl cinnamate
Catalog No.:BCN5044
CAS No.:103-36-6
Phase II study of MLN8237 (Alisertib) in advanced/metastatic sarcoma.[Pubmed:27502708]
Ann Oncol. 2016 Oct;27(10):1855-60.
BACKGROUND: Aurora kinase A (AURKA) is commonly overexpressed in sarcoma. The inhibition of AURKA by shRNA or by a specific AURKA inhibitor blocks in vitro proliferation of multiple sarcoma subtypes. MLN8237 (Alisertib) is a novel oral adenosine triphosphate-competitive AURKA inhibitor. PATIENTS AND METHODS: This Cancer Therapy Evaluation Program-sponsored phase II study of alisertib was conducted through the Alliance for Clinical Trials in Oncology (A091102). Patients were enrolled into histology-defined cohorts: (i) liposarcoma, (ii) leiomyosarcoma, (iii) undifferentiated sarcoma, (iv) malignant peripheral nerve sheath tumor, or (v) other. Treatment was alisertib 50 mg PO b.i.d. d1-d7 every 21 days. The primary end point was response rate; progression-free survival (PFS) was secondary. One response in the first 9 patients expanded enrollment in a cohort to 24 using a Simon two-stage design. RESULTS: Seventy-two patients were enrolled at 24 sites [12 LPS, 10 LMS, 11 US, 10 malignant peripheral nerve sheath tumor (MPNST), 29 Other]. The median age was 55 years; 54% were male; 58%/38%/4% were ECOG PS 0/1/2. One PR expanded enrollment to the second stage in the other sarcoma cohort. The histology-specific cohorts ceased at the first stage. There were two confirmed PRs in the other cohort (both angiosarcoma) and one unconfirmed PR in dedifferentiated chondrosarcoma. Twelve-week PFS was 73% (LPS), 44% (LMS), 36% (US), 60% (MPNST), and 38% (Other). Grade 3-4 adverse events: oral mucositis (12%), anemia (14%), platelet count decreased (14%), leukopenia (22%), and neutropenia (42%). CONCLUSIONS: Alisertib was well tolerated. Occasional responses, yet prolonged stable disease, were observed. Although failing to meet the primary RR end point, PFS was promising. TRIAL REGISTRATION ID: NCT01653028.
A phase 2 study of alisertib (MLN8237) in recurrent or persistent uterine leiomyosarcoma: An NRG Oncology/Gynecologic Oncology Group study 0231D.[Pubmed:28094040]
Gynecol Oncol. 2017 Jan;144(1):96-100.
OBJECTIVE: This two-stage Phase II study assessed the activity of single agent alisertib in patients with recurrent/persistent uterine leiomyosarcoma (uLMS). METHODS: Eligibility criteria included histologically-confirmed, recurrent or persistent uLMS, age>/=18, 1-2 prior cytotoxic regimens, and RECIST version 1.1 measurable disease. The primary objective of the study was to evaluate the efficacy of alisertib through the frequency of patients with objective tumor responses and the frequency who survived event-free for at least 6months (EFS6). The endpoints for EFS were RECIST progression, death, or beginning a subsequent therapy. The null hypothesis jointly specified the probability of a patient experiencing a tumor response to less than or equal to 5% and the probability of a patient surviving event-free for at least 6months to less than or equal to 20%. A two-stage design was used with a target accrual of 23 patients for stage 1 and 47 pts. cumulative for stage 2. Confidence intervals do not correct for multiplicity. RESULTS: Twenty-three patients were enrolled with two patients excluded on central histology review, yielding 21 eligible patients. Median age was 61years. Prior treatment was either 1 cytotoxic regimen (71.4%) or 2 (28.6%). The most common treatment related AEs (grade 3 or worse) were anemia Hensley et al. (2008a) , leukopenia Hensley et al. (2008b) , neutropenia Maki et al. (2007) , thrombocytopenia Huang et al. (2012) , mucositis Hensley et al. (2008a) , diarrhea Huang et al. (2012) , and palmer-planter syndrome Zivanovic et al. (2012) . There were no objective responses (0%; 90% CI: 0-10.4%). Best response was stable disease (38.1%); 12 patients had progressive disease (57.1%). EFS6 was 0% (90% CI: 0-10.4%). Median PFS and OS were 1.7 (90% CI: 1.4-3.2) and 14.5months (90% CI: 7.6 - NA), respectively. CONCLUSION: Alisertib did not demonstrate clinically meaningful single agent activity in previously treated uLMS.
Open-label, multicenter, phase 1 study of alisertib (MLN8237), an aurora A kinase inhibitor, with docetaxel in patients with solid tumors.[Pubmed:27192055]
Cancer. 2016 Aug 15;122(16):2524-33.
BACKGROUND: This study was designed to determine the safety, tolerability, and pharmacokinetics (PK) of alisertib (MLN8237) in combination with docetaxel and to identify a recommended dose for the combination. METHODS: Adults with metastatic cancer were treated on 21-day cycles with alisertib (10, 20, 30, or 40 mg) twice daily on days 1 to 7 or days 1 to 5 and with docetaxel (75 or 60 mg/m(2) ) on day 1. The primary objectives were to assess the safety and tolerability of the combination and to determine the recommended phase 2 dose (RP2D) for future studies. Secondary objectives included an efficacy assessment and PK analyses of docetaxel and alisertib. RESULTS: Forty-one patients participated. Eight dose levels were explored with various doses of alisertib and docetaxel. The dose-limiting toxicities were neutropenic fever, neutropenia without fever, stomatitis, and urinary tract infection. The RP2D of this combination was 20 mg of alisertib twice daily on days 1 to 7 and intravenous docetaxel at 75 mg/m(2) on day 1 in 21-day cycles. Eight of the 28 patients (29%) who were efficacy-evaluable had objective responses. These included 1 complete response in a patient with bladder cancer, 6 partial responses in patients with castration-resistant prostate cancer, and 1 partial response in a patient with angiosarcoma. Concomitant administration of alisertib did not produce any clinically meaningful change in docetaxel PK. CONCLUSIONS: Alisertib at 20 mg twice daily on days 1 to 7 with intravenous docetaxel at 75 mg/m(2) on day 1 in a 21-day cycle was well tolerated, and the combination demonstrated antitumor activity. Cancer 2016;122:2524-33. (c) 2016 American Cancer Society.


