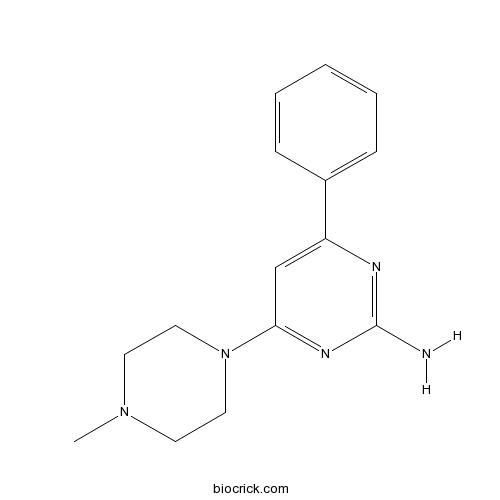
VUF 10460Selective H4 agonist CAS# 1028327-66-3 |
- VU 0361737
Catalog No.:BCC4596
CAS No.:1161205-04-4
- Ifenprodil Tartrate
Catalog No.:BCC4589
CAS No.:23210-58-4
- VU 0364770
Catalog No.:BCC4597
CAS No.:61350-00-3
- MK-801 (Dizocilpine)
Catalog No.:BCC4591
CAS No.:77086-21-6
- Latrepirdine
Catalog No.:BCC4541
CAS No.:97657-92-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1028327-66-3 | SDF | Download SDF |
| PubChem ID | 25129523 | Appearance | Powder |
| Formula | C15H19N5 | M.Wt | 269.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 36 mg/mL (133.66 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-(4-methylpiperazin-1-yl)-6-phenylpyrimidin-2-amine | ||
| SMILES | CN1CCN(CC1)C2=NC(=NC(=C2)C3=CC=CC=C3)N | ||
| Standard InChIKey | NIJGWJIOMPHDBP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H19N5/c1-19-7-9-20(10-8-19)14-11-13(17-15(16)18-14)12-5-3-2-4-6-12/h2-6,11H,7-10H2,1H3,(H2,16,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective histamine H4 receptor agonist; displays 50-fold selectivity for the rat H4 receptor over the H3 subtype (pKi values are 5.75 and 7.46 for rat H3 and H4 receptors respectively). Also exhibits affinity for the human H4 receptor (pKi = 8.22). |

VUF 10460 Dilution Calculator

VUF 10460 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7128 mL | 18.5639 mL | 37.1278 mL | 74.2556 mL | 92.8195 mL |
| 5 mM | 0.7426 mL | 3.7128 mL | 7.4256 mL | 14.8511 mL | 18.5639 mL |
| 10 mM | 0.3713 mL | 1.8564 mL | 3.7128 mL | 7.4256 mL | 9.2819 mL |
| 50 mM | 0.0743 mL | 0.3713 mL | 0.7426 mL | 1.4851 mL | 1.8564 mL |
| 100 mM | 0.0371 mL | 0.1856 mL | 0.3713 mL | 0.7426 mL | 0.9282 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydrocinchonamine
Catalog No.:BCN5841
CAS No.:10283-68-8
- RuBi-GABA
Catalog No.:BCC6012
CAS No.:1028141-88-9
- [D-p-Cl-Phe6,Leu17]-VIP
Catalog No.:BCC5968
CAS No.:102805-45-8
- GYKI 52466 dihydrochloride
Catalog No.:BCC7072
CAS No.:102771-26-6
- Levetiracetam
Catalog No.:BCC1056
CAS No.:102767-28-2
- PSB 0788
Catalog No.:BCC7599
CAS No.:1027513-54-7
- H-Gln(Trt)-OH
Catalog No.:BCC2919
CAS No.:102747-84-2
- VX-222 (VCH-222, Lomibuvir)
Catalog No.:BCC2108
CAS No.:1026785-59-0
- Labd-13-ene-8,15-diol
Catalog No.:BCN5840
CAS No.:10267-31-9
- LB-100
Catalog No.:BCC5532
CAS No.:1026680-07-8
- RO-3
Catalog No.:BCC7548
CAS No.:1026582-88-6
- SC-10
Catalog No.:BCC6643
CAS No.:102649-79-6
- BEZ235 Tosylate
Catalog No.:BCC1416
CAS No.:1028385-32-1
- D-Pinitol
Catalog No.:BCN5842
CAS No.:10284-63-6
- Mulberroside A
Catalog No.:BCN6343
CAS No.:102841-42-9
- Mulberroside C
Catalog No.:BCN6344
CAS No.:102841-43-0
- Moracin P
Catalog No.:BCN3289
CAS No.:102841-46-3
- Ganolactone B
Catalog No.:BCN2872
CAS No.:1028449-53-7
- MLN8237 (Alisertib)
Catalog No.:BCC2166
CAS No.:1028486-01-2
- Intermedine
Catalog No.:BCN1997
CAS No.:10285-06-0
- Lycopsamine
Catalog No.:BCN1999
CAS No.:10285-07-1
- 17-Hydroxy sprengerinin C
Catalog No.:BCN2755
CAS No.:1029017-75-1
- Pexidartinib (PLX3397)
Catalog No.:BCC6405
CAS No.:1029044-16-3
- MDL 73005EF hydrochloride
Catalog No.:BCC6636
CAS No.:102908-60-1
Discovery of S-(2-guanidylethyl)-isothiourea (VUF 8430) as a potent nonimidazole histamine H4 receptor agonist.[Pubmed:17154494]
J Med Chem. 2006 Nov 16;49(23):6650-1.
During an in-house database screen, we identified S-(2-guanidylethyl)-isothiourea as a high affinity agonist for the histamine H4 receptor, with a 33-fold selectivity over the histamine H3 receptor and negligible affinity for the other histamine receptor subtypes. This nonimidazole ligand is introduced as a useful and complementary pharmacological tool that enables further unraveling of the physiological roles of the H4 receptor.
Effects of a new antiallergic drug, VUF-K-8788, on infiltration of lung parenchyma by eosinophils in guinea pigs and eosinophil-adhesion to human umbilical vein endothelial cells (HUVEC).[Pubmed:11642316]
Biol Pharm Bull. 2001 Oct;24(10):1127-32.
Airway inflammation and reversible airway obstruction are hallmarks of bronchial asthma. In this study, we investigated the effects of a new antiallergic drug, 7-[3-[4-(2-quinolinylmethyl)-1-piperazinyl]-propoxy]-2,3-dihydro-4H-1,4-benzothia zin-3-one (VUF-K-8788), on histopathological changes in lung parenchyma of guinea pigs during late-phase asthmatic reaction (LAR), and on eosinophil-adhesion to human umbilical vein endothelial cells (HUVEC). Repeated exposure to ovalbumin of sensitized guinea pigs induced inflammatory phenomena such as hyperplasia of airway epithelial cells, perivascular edema and infiltration of lung parenchyma by eosinophils. VUF-K-8788 inhibited these histopathological phenomena at 10 mg/kg p.o. Moreover, the eosinophil-adherence to HUVEC was inhibited by VUF-K-8788 at the concentration of 10-30 microM. In conclusion, this inhibitory effect of VUF-K-8788 on eosinophil-adherence might contribute to the prevention of LAR and infiltration by eosinophils in the experimental asthmatic model in guinea pigs.
Pharmacological characterization of the new histamine H4 receptor agonist VUF 8430.[Pubmed:19413569]
Br J Pharmacol. 2009 May;157(1):34-43.
BACKGROUND AND PURPOSE: We compare the pharmacological profiles of a new histamine H4 receptor agonist 2-(2-guanidinoethyl)isothiourea (VUF 8430) with that of a previously described H4 receptor agonist, 4-methylhistamine. EXPERIMENTAL APPROACH: Radioligand binding and functional assays were performed using histamine H4 receptors expressed in mammalian cell lines. Compounds were also evaluated ex vivo in monocyte-derived dendritic cells endogenously expressing H4 receptors and in vivo in anaesthetized rats for gastric acid secretion activity. KEY RESULTS: Both VUF 8430 and 4-methylhistamine were full agonists at human H4 receptors with lower affinity at rat and mouse H4 receptors. Both compounds induced chemotaxis of monocyte-derived dendritic cells. VUF 8430 also showed reasonable affinity and was a full agonist at the H3 receptor. Agmatine is a metabolite of arginine, structurally related to VUF 8430, and was a H4 receptor agonist with micromolar affinity. At histamine H3 receptors, agmatine was a full agonist, whereas 4-methylhistamine was an agonist only at high concentrations. Both VUF 8430 and agmatine were inactive at H1 and H2 receptors, whereas 4-methylhistamine is as active as histamine at H2 receptors. In vivo, VUF 8430 only caused a weak secretion of gastric acid mediated by H2 receptors, whereas 4-methylhistamine, dimaprit, histamine and amthamine, at equimolar doses, induced 2.5- to 6-fold higher output than VUF 8430. CONCLUSIONS AND IMPLICATIONS: Our results suggest complementary use of 4-methylhistamine and VUF 8430 as H4 receptor agonists. Along with H4 receptor antagonists, both agonists can serve as useful pharmacological tools in studies of histamine H4 receptors.
Selective histamine H(3) and H(4) receptor agonists exert opposite effects against the gastric lesions induced by HCl in the rat stomach.[Pubmed:21839070]
Eur J Pharmacol. 2011 Nov 1;669(1-3):121-7.
The present study investigated the role of histamine H(3) and H(4) receptors in gastric mucosal defense, by the use of selective ligands. Firstly, the affinities of several histaminergic agonists for the rat histamine H(3) and H(4) receptors were checked in HEK 293T cells transfected with either receptor subtype. Next, functional activities were determined in conscious rat against the ulcerogenic effect of 0.6N HCl. Radioligand binding studies showed that immethridine and methimepip were the most selective agonists at rat H(3) receptors, whereas VUF10460 displayed approximately a 50-fold selectivity for the rat H(4) receptor over the H(3) receptor. In conscious rats, immethridine and methimepip significantly reduced (66% and 48% inhibition, respectively) the gastric lesions induced by HCl; the effect of immethridine was antagonized by the H(3) receptor antagonist A-331440, but not by the H(4) receptor antagonist JNJ7777120. The mixed H(3)/H(4) receptor agonist immepip induced a significant aggravation of HCl damage, which was prevented by JNJ7777120; HCl-induced lesions were also significantly enhanced by the H(4) receptor agonists VUF10460 and VUF8430; however, this effect was not modified by JNJ7777120. Overall, this study indicates that, whereas the histamine H(3) receptor is involved in the protection of rat stomach against concentrated HCl, the functional role of the H(4) receptor is still to be defined, although selective agonists induce proulcerogenic effects under HCl challenge. Finally, the species-dependent variations in affinity and receptor selectivity observed for most ligands need to be carefully addressed in the pharmacological characterization of histamine H(3) and H(4) receptor functions in vivo.
Structure-activity studies on a series of a 2-aminopyrimidine-containing histamine H4 receptor ligands.[Pubmed:18811133]
J Med Chem. 2008 Oct 23;51(20):6571-80.
A series of 2-aminopyrimidines was synthesized as ligands of the histamine H4 receptor (H4R). Working in part from a pyrimidine hit that was identified in an HTS campaign, SAR studies were carried out to optimize the potency, which led to compound 3, 4- tert-butyl-6-(4-methylpiperazin-1-yl)pyrimidin-2-ylamine. We further studied this compound by systematically modifying the core pyrimidine moiety, the methylpiperazine at position 4, the NH2 at position 2, and positions 5 and 6 of the pyrimidine ring. The pyrimidine 6 position benefited the most from this optimization, especially in analogs in which the 6- tert-butyl was replaced with aromatic and secondary amine moieties. The highlight of the optimization campaign was compound 4, 4-[2-amino-6-(4-methylpiperazin-1-yl)pyrimidin-4-yl]benzonitrile, which was potent in vitro and was active as an anti-inflammatory agent in an animal model and had antinociceptive activity in a pain model, which supports the potential of H 4R antagonists in pain.
Rotationally constrained 2,4-diamino-5,6-disubstituted pyrimidines: a new class of histamine H4 receptor antagonists with improved druglikeness and in vivo efficacy in pain and inflammation models.[Pubmed:18817367]
J Med Chem. 2008 Oct 23;51(20):6547-57.
A new structural class of histamine H 4 receptor antagonists (6-14) was designed based on rotationally restricted 2,4-diaminopyrimidines. Series compounds showed potent and selective in vitro H 4 antagonism across multiple species, good CNS penetration, improved PK properties compared to reference H 4 antagonists, functional H 4 antagonism in cellular and in vivo pharmacological assays, and in vivo anti-inflammatory and antinociceptive efficacy. One compound, 10 (A-943931), combined the best features of the series in a single molecule and is an excellent tool compound to probe H 4 pharmacology. It is a potent H 4 antagonist in functional assays across species (FLIPR Ca (2+) flux, K b < 5.7 nM), has high (>190x) selectivity for H 4, and combines good PK in rats and mice (t 1/2 of 2.6 and 1.6 h, oral bioavailability of 37% and 90%) with anti-inflammatory activity (ED 50 = 37 micromol/kg, mouse) and efficacy in pain models (thermal hyperalgesia, ED 50 = 72 micromol/kg, rat).


