SC-10Protein kinase C activator CAS# 102649-79-6 |
- Sulfacetamide Sodium
Catalog No.:BCC4383
CAS No.:127-56-0
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- Doxorubicin
Catalog No.:BCC2082
CAS No.:23214-92-8
- Divalproex Sodium
Catalog No.:BCC4379
CAS No.:76584-70-8
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 102649-79-6 | SDF | Download SDF |
| PubChem ID | 5175 | Appearance | Powder |
| Formula | C17H22ClNO2S | M.Wt | 339.88 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO | ||
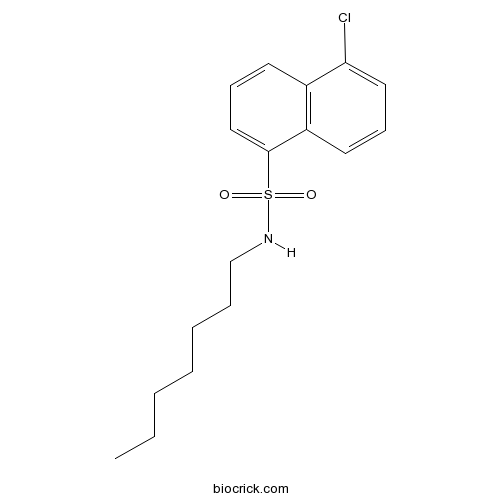
| Chemical Name | 5-chloro-N-heptylnaphthalene-1-sulfonamide | ||
| SMILES | CCCCCCCNS(=O)(=O)C1=CC=CC2=C1C=CC=C2Cl | ||
| Standard InChIKey | MJMJERJFCHYXEM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H22ClNO2S/c1-2-3-4-5-6-13-19-22(20,21)17-12-8-9-14-15(17)10-7-11-16(14)18/h7-12,19H,2-6,13H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Activator of protein kinase C. |

SC-10 Dilution Calculator

SC-10 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9422 mL | 14.7111 mL | 29.4221 mL | 58.8443 mL | 73.5554 mL |
| 5 mM | 0.5884 mL | 2.9422 mL | 5.8844 mL | 11.7689 mL | 14.7111 mL |
| 10 mM | 0.2942 mL | 1.4711 mL | 2.9422 mL | 5.8844 mL | 7.3555 mL |
| 50 mM | 0.0588 mL | 0.2942 mL | 0.5884 mL | 1.1769 mL | 1.4711 mL |
| 100 mM | 0.0294 mL | 0.1471 mL | 0.2942 mL | 0.5884 mL | 0.7356 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SC-9
Catalog No.:BCC6646
CAS No.:102649-78-5
- Pantoprazole
Catalog No.:BCC5432
CAS No.:102625-70-7
- Saprorthoquinone
Catalog No.:BCN3147
CAS No.:102607-41-0
- Ganoderic acid L
Catalog No.:BCN8204
CAS No.:102607-24-9
- Methyl lucidente G
Catalog No.:BCN8269
CAS No.:102607-20-5
- R788 disodium
Catalog No.:BCC3695
CAS No.:1025687-58-4
- 2,3,23-Trihydroxy-12-oleanen-28-oic acid
Catalog No.:BCN1638
CAS No.:102519-34-6
- (-)-Huperzine A
Catalog No.:BCN1057
CAS No.:102518-79-6
- SGI-1776 free base
Catalog No.:BCC2232
CAS No.:1025065-69-3
- Periglaucine B
Catalog No.:BCN7053
CAS No.:1025023-05-5
- Periglaucine A
Catalog No.:BCN5839
CAS No.:1025023-04-4
- GK921
Catalog No.:BCC8057
CAS No.:1025015-40-0
- RO-3
Catalog No.:BCC7548
CAS No.:1026582-88-6
- LB-100
Catalog No.:BCC5532
CAS No.:1026680-07-8
- Labd-13-ene-8,15-diol
Catalog No.:BCN5840
CAS No.:10267-31-9
- VX-222 (VCH-222, Lomibuvir)
Catalog No.:BCC2108
CAS No.:1026785-59-0
- H-Gln(Trt)-OH
Catalog No.:BCC2919
CAS No.:102747-84-2
- PSB 0788
Catalog No.:BCC7599
CAS No.:1027513-54-7
- Levetiracetam
Catalog No.:BCC1056
CAS No.:102767-28-2
- GYKI 52466 dihydrochloride
Catalog No.:BCC7072
CAS No.:102771-26-6
- [D-p-Cl-Phe6,Leu17]-VIP
Catalog No.:BCC5968
CAS No.:102805-45-8
- RuBi-GABA
Catalog No.:BCC6012
CAS No.:1028141-88-9
- Dihydrocinchonamine
Catalog No.:BCN5841
CAS No.:10283-68-8
- VUF 10460
Catalog No.:BCC6285
CAS No.:1028327-66-3
Biodesulfurization of diesel oil in oil-water two phase reaction system by Gordonia sp. SC-10.[Pubmed:30915612]
Biotechnol Lett. 2019 Mar 26. pii: 10.1007/s10529-019-02663-9.
OBJECTIVES: Different sulfur contents of diesel oils were used for biodesulfurization to study the desulfurization capacity of Gordonia sp. SC-10 in oil-water two-phase reaction system. RESULTS: Gordonia sp. SC-10 showed great properties in desulfurizing diesel oil with different sulfur contents. This bacterium could decrease sulfur contents in different diesel oils from 194.7 +/- 3.7 to 30.4 +/- 0.5 mg/l and from 3035.3 +/- 23.8 to 1792.8 +/- 48.9 mg/l, respectively. Furthermore, this bacterium could desulfurize broad range of organosulfur compounds and had strong desulfurization activity against alkylated DBTs. For low-sulfur diesel oil, sulfur could be removed from 10.2 +/- 0.1 to 5.0 +/- 0.1 mg/l. CONCLUSIONS: The newly isolated bacteria Gordonia sp. SC-10 showed a good performance in desulfurizing diesel oils, and it might be a useful desulfurizing biocatalyst to enable the industrialized application of biodesulfurization process.
Fungal biomass as biosorbent for the removal of Acid Blue 161 dye in aqueous solution.[Pubmed:27909927]
Environ Sci Pollut Res Int. 2017 Feb;24(4):4200-4209.
Physical and thermal treatment was used to inactivate Trametes sp. SC-10 fungus. The resulting biomass was named BTV, characterized by analytical techniques such as SEM, EDX, FTIR, BET, and Barrett-Joyner-Halenda (BJH) model. pH, kinetic, and equilibrium adsorption studies with the Acid Blue 161 (AB-161) dye were investigated at 303.15 K. The kinetics of the biosorption process were examined at 600.00 and 1300 mg L(-1), using pseudo-first-order, pseudo-second-order, and Avrami fractional-order models. The maximum biosorption capacity of BTV for AB-161 dye was 221.6 mg g(-1). Considering the biosorption data and the functional groups of BTV, it can be inferred that the sorption mechanism of AB-161 is regulated by electrostatic interactions between ionized dye molecules and negative charges on BTV in an aqueous solution. Finally, the BTV was tested with a simulated effluent with 89.47% efficiency, presenting the BTV as a biosorbent for real effluents polluted with dyes.
Comparative evaluation of marginal leakage of provisional crowns cemented with different temporary luting cements: In vitro study.[Pubmed:27134427]
J Indian Prosthodont Soc. 2016 Jan-Mar;16(1):42-8.
BACKGROUND OR STATEMENT OF PROBLEM: As, the longevity of provisional restorations is related to, a perfect adaptation and a strong, long-term union between restoration and teeth structures, therefore, evaluation of marginal leakage of provisional restorative materials luted with cements using the standardized procedures is essential. AIMS AND OBJECTIVES: To compare the marginal leakage of the provisional crowns fabricated from Autopolymerizing acrylic resin crowns and bisphenol A-glycidyl dimethacrylate (BIS-GMA) resin crowns. To compare the marginal leakage of the provisional crowns fabricated from autopolymerizing acrylic resin crowns and BIS-GMA resin crowns cemented with different temporary luting cements. To compare the marginal leakage of the provisional crowns fabricated from autopolymerizing acrylic resin (SC-10) crowns cemented with different temporary luting cements. To compare the marginal leakage of the provisional crowns fabricated from BIS-GMA resin crowns (Protemp 4) cemented with different temporary luting cements. METHODOLOGY: Freshly extracted 60 maxillary premolars of approximately similar dimensions were mounted in dental plaster. Tooth reduction with shoulder margin was planned to use a customized handpiece-holding jig. Provisional crowns were prepared using the wax pattern fabricated from computer aided designing/computer aided manufacturing milling machine following the tooth preparation. Sixty provisional crowns were made, thirty each of SC-10 and Protemp 4 and were then cemented with three different luting cements. Specimens were thermocycled, submerged in a 2% methylene blue solution, then sectioned and observed under a stereomicroscope for the evaluation of marginal microleakage. A five-level scale was used to score dye penetration in the tooth/cement interface and the results of this study was analyzed using the Chi-square test, Mann-Whitney U-test, Kruskal-Wallis H-test and the results were statistically significant P < 0.05 the power of study - 80%. RESULTS: Marginal leakage was significant in both provisional crowns cemented with three different luting cements along the axial walls of teeth (P < 0.05) confidence interval - 95%. CONCLUSION: The temporary cements with eugenol showed more microleakage than those without eugenol. SC-10 crowns showed more microleakage compared to Protemp 4 crowns. SC-10 crowns cemented with Kalzinol showed maximum microleakage and Protemp 4 crowns cemented with HY bond showed least microleakage.
N-(6-phenylhexyl)-5-chloro-1-naphthalenesulfonamide, a novel activator of protein kinase C.[Pubmed:3756133]
Biochemistry. 1986 Jul 29;25(15):4179-84.
Naphthalenesulfonamide derivatives were used to study the mechanism of regulation of Ca2+-dependent smooth muscle myosin light chain phosphorylation catalyzed by Ca2+-activated, phospholipid-dependent protein kinase (protein kinase C) and myosin light chain kinase. Derivatives such as N-(6-phenylhexyl)-5-chloro-1-naphthalenesulfonamide (SC-9), with a hydrophobic residue at the end of a hydrocarbon chain, stimulated Ca2+-activated, phospholipid-dependent myosin light chain phosphorylation in a Ca2+-dependent fashion. There was no significant effect of these compounds on Ca2+-calmodulin (CaM) dependent myosin light chain phosphorylation. On the other hand, derivatives with the guanidino or amino residue at the same position had an inhibitory effect on both Ca2+-phospholipid- and Ca2+-CaM-dependent myosin light chain phosphorylation. These observations suggest that activation of Ca2+-activated, phospholipid-dependent myosin light chain phosphorylation by naphthalenesulfonamide derivatives depends on the chemical structure at the end of hydrocarbon chain of each compound. SC-9 was similar to phosphatidylserine with regard to activation, and the apparent Km values for Ca2+ of the enzyme with this compound and phosphatidylserine were 40 microM and 80 microM, respectively. Kinetic analysis indicated that 12-O-tetradecanoylphorbol 13-acetate increased the affinity of the enzyme with SC-9 for calcium ion. However, kinetic constants revealed that the Km value of protein kinase C activated by SC-9 for substrate myosin light chain was 5.8 microM, that is, about 10 times lower than that of the enzyme with phosphatidylserine, and that the Vmax value with SC-9 was 0.13 nmol X min-1, that is, 3-fold smaller than that seen with phosphatidylserine.(ABSTRACT TRUNCATED AT 250 WORDS)


