Ethyl palmitoleateCAS# 56219-10-4 |
Quality Control & MSDS
Number of papers citing our products

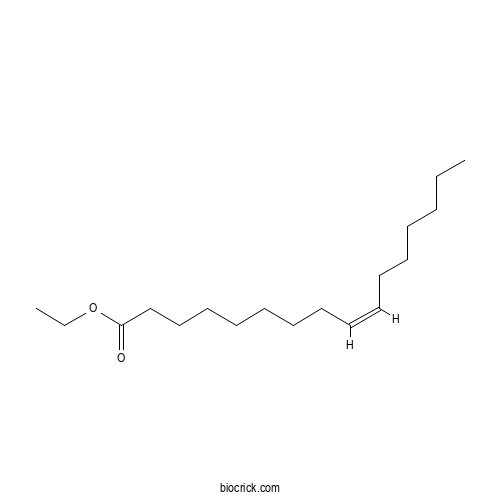
Chemical structure

3D structure
| Cas No. | 56219-10-4 | SDF | Download SDF |
| PubChem ID | 6436624.0 | Appearance | Powder |
| Formula | C18H34O2 | M.Wt | 282.47 |
| Type of Compound | Aliphatics | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | ethyl (Z)-hexadec-9-enoate | ||
| SMILES | CCCCCCC=CCCCCCCCC(=O)OCC | ||
| Standard InChIKey | JELGPLUONQGOHF-KTKRTIGZSA-N | ||
| Standard InChI | InChI=1S/C18H34O2/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20-4-2/h9-10H,3-8,11-17H2,1-2H3/b10-9- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ethyl palmitoleate Dilution Calculator

Ethyl palmitoleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5402 mL | 17.701 mL | 35.402 mL | 70.804 mL | 88.505 mL |
| 5 mM | 0.708 mL | 3.5402 mL | 7.0804 mL | 14.1608 mL | 17.701 mL |
| 10 mM | 0.354 mL | 1.7701 mL | 3.5402 mL | 7.0804 mL | 8.8505 mL |
| 50 mM | 0.0708 mL | 0.354 mL | 0.708 mL | 1.4161 mL | 1.7701 mL |
| 100 mM | 0.0354 mL | 0.177 mL | 0.354 mL | 0.708 mL | 0.885 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nonadecanoic acid
Catalog No.:BCX1376
CAS No.:646-30-0
- Methyl heptadecanoate
Catalog No.:BCX1375
CAS No.:1731-92-6
- Heptadecanoic acid
Catalog No.:BCX1374
CAS No.:506-12-7
- Pentadecanoic acid
Catalog No.:BCX1373
CAS No.:1002-84-2
- Methyl tridecanoate
Catalog No.:BCX1372
CAS No.:1731-88-0
- Tridecanoic acid
Catalog No.:BCX1371
CAS No.:638-53-9
- Octadec-11-enoic acid
Catalog No.:BCX1370
CAS No.:693-72-1
- Cis-11-Eicosenoic acid
Catalog No.:BCX1369
CAS No.:5561-99-9
- Elaidic acid methyl ester
Catalog No.:BCX1368
CAS No.:1937-62-8
- Myristoleic acid
Catalog No.:BCX1367
CAS No.:544-64-9
- Ethyl (2E,4Z)-deca-2,4-dienoate
Catalog No.:BCX1366
CAS No.:3025-30-7
- Morroniaglycone
Catalog No.:BCX1365
CAS No.:1644061-02-8
- Palmitoleic acid
Catalog No.:BCX1378
CAS No.:373-49-9
- Arachidic acid
Catalog No.:BCX1379
CAS No.:506-30-9
- Ethyl Arachidonate
Catalog No.:BCX1380
CAS No.:1808-26-0
- Methyl arachidonate
Catalog No.:BCX1381
CAS No.:2566-89-4
- Ethyl oleate
Catalog No.:BCX1382
CAS No.:111-62-6
- Docosahexaenoic acid ethyl ester
Catalog No.:BCX1383
CAS No.:84494-72-4
- Docosahexaenoic acid methyl ester
Catalog No.:BCX1384
CAS No.:2566-90-7
- Eicosapentaenoic acid ethyl ester
Catalog No.:BCX1385
CAS No.:86227-47-6
- Methyl Eicosapentaenoate
Catalog No.:BCX1386
CAS No.:2734-47-6
- Linoleic acid sodium salt
Catalog No.:BCX1387
CAS No.:822-17-3
- γ-Linolenic acid
Catalog No.:BCX1388
CAS No.:506-26-3
- Arundoin
Catalog No.:BCX1389
CAS No.:4555-56-0
Phytocompounds of Nigella sativa seeds extract and their neuroprotective potential via EGR1 receptor inhibition: A molecular docking study.[Pubmed:38454971]
Narra J. 2023 Aug;3(2):e173.
Bioactivity of Nigella sativa seed extract has the potential as a neuro-protector, offering its promising utility in the clinical setting for brain injury management. This study aimed to identify the phytocompounds contained in the extract of N. sativa seeds and further screen their respective neuronal anti-inflammatory activities in silico. The extract of N. sativa seeds was prepared through successive maceration using non-polar to polar solvents (n-hexane and ethanol, respectively). The phytocompounds in the ethanolic extract were initially identified through qualitative analysis and further analyzed with gas chromatography-mass spectrometry (GC-MS). The spectral data were compared with the compound library for identification. The identified phytocompounds were then simulated computationally for their binding affinities toward the active pocket of early growth response-1 (EGR1) receptor (PDB: 14r2a). We found that the ethanolic extract of N. sativa seeds were predominantly constituted of hexadecanoic acid, ethyl ester (17.15%); linoleic acid ethyl ester (15.0%); octadecanoic acid (13.26%); and ethyl oleate (10.38%). The binding affinity of the phytocompounds ranged from -7.49 kcal/mol (mEthyl palmitoleate) to -14.31 kcal/mol (9-hexadecanoic acid, methyl ester), with 12 compounds having binding affinity < -10 kcal/mol. In conclusion, ethanolic extract of N. sativa seeds are rich with fatty acids that have active as anti-inflammatory and may exert neuronal protection by inhibiting EGR1 receptor. Studies using animal models to confirm the activity are warranted.
New insights into changing honey bee (Apis mellifera) immunity molecules pattern and fatty acid esters, in responses to Ascosphaera apis infection.[Pubmed:38065241]
J Invertebr Pathol. 2024 Feb;202:108028.
Monitoring of metabolite changes could provide valuable insights into disturbances caused by an infection and furthermore, could be used to define the status of an organism as healthy or diseased and define what could be defensive elements against the infection. The present investigation conducted a gas chromatography-mass spectrometry (GC/MS) for haemolymph of larval honey bees (Apis mellifera L.) infected with the fungal pathogen Ascosphaera apis in comparison with control haemolymph non-infected insects. Results revealed that the pathogen caused a general disturbance of metabolites detected in the haemolymph of the honey bee. The majority of metabolites identified before and after infection were fatty acid esters. The disease caused an elevation in levels of methyl oleate, methyl palmitate, and methyl stearate, respectively. Further, the disease drove to the disappearance of mEthyl palmitoleate, and methyl laurate. Conversely, methyl linolelaidate, and ethyl oleate were identified only in infected larvae. A high reduction in diisooctyl phthalate was recorded after the infection. Interestingly, antimicrobial activities were confirmed for haemolymph of infected honey bee larvae. In spite of the presence of some previously known bioactive compounds in healthy larvae there were no antimicrobial activities.
Poria cocos (Schw.) Wolf, a Traditional Chinese Edible Medicinal Herb, Promotes Neuronal Differentiation, and the Morphological Maturation of Newborn Neurons in Neural Stem/Progenitor Cells.[Pubmed:38005201]
Molecules. 2023 Nov 8;28(22):7480.
Neurogenesis in the adult brain comprises the entire set of events of neuronal development. It begins with the division of precursor cells to form a mature, integrated, and functioning neuronal network. Adult neurogenesis is believed to play an important role in animals' cognitive abilities, including learning and memory. In the present study, significant neuronal differentiation-promoting activity of 80% (v/v) ethanol extract of P. cocos (EEPC) was found in Neuro-2a cells and mouse cortical neural stem/progenitor cells (NSPCs). Subsequently, a total of 97 compounds in EEPC were identified by UHPLC-Q-Exactive-MS/MS. Among them, four major compounds-Adenosine; Choline; Ethyl palmitoleate; and L-(-)-arabinitol-were further studied for their neuronal differentiation-promoting activity. Of which, choline has the most significant neuronal differentiation-promoting activity, indicating that choline, as the main bioactive compound in P. cocos, may have a positive effect on learning and memory functions. Compared with similar research literature, this is the first time that the neuronal differentiation-promoting effects of P. cocos extract have been studied.
Attraction and Electrophysiological Response to Identified Rectal Gland Volatiles in Bactrocera frauenfeldi (Schiner).[Pubmed:32168881]
Molecules. 2020 Mar 11;25(6):1275.
Bactrocera frauenfeldi (Schiner) (Diptera: Tephritidae) is a polyphagous fruit fly pest species that is endemic to Papua New Guinea and has become established in several Pacific Islands and Australia. Despite its economic importance for many crops and the key role of chemical-mediated sexual communication in the reproductive biology of tephritid fruit flies, as well as the potential application of pheromones as attractants, there have been no studies investigating the identity or activity of rectal gland secretions or emission profiles of this species. The present study (1) identifies the chemical profile of volatile compounds produced in rectal glands and released by B. frauenfeldi, (2) investigates which of the volatile compounds elicit an electroantennographic or electropalpographic response, and (3) investigates the potential function of glandular emissions as mate-attracting sex pheromones. Rectal gland extracts and headspace collections from sexually mature males and females of B. frauenfeldi were analysed by gas chromatography-mass spectrometry. Male rectal glands contained (E,E)-2-ethyl-8-methyl-1,7-dioxaspiro [5.5]undecane as a major component and (E,E)-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane as a moderate component. Minor components included palmitoleic acid, palmitic acid, and ethyl oleate. In contrast, female rectal glands contained (E,E)-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane and ethyl laurate as major components, ethyl myristate and Ethyl palmitoleate as moderate components, and 18 minor compounds including amides, esters, and spiroacetals. Although fewer compounds were detected from the headspace collections of both males and females than from the gland extractions, most of the abundant chemicals in the rectal gland extracts were also detected in the headspace collections. Gas chromatography coupled electroantennographic detection found responses to (E,E)-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane from the antennae of both male and female B. frauenfeldi. Responses to (E,E)-2-ethyl-8-methyl-1,7-dioxaspiro[5.5]undecane were elicited from the antennae of females but not males. The two spiroacetals also elicited electropalpographic responses from both male and female B. frauenfeldi. Ethyl caprate and methyl laurate, found in female rectal glands, elicited responses in female antennae and palps, respectively. Y-maze bioassays showed that females were attracted to the volatiles from male rectal glands but males were not. Neither males nor females were attracted to the volatiles from female rectal glands. Our findings suggest (E,E)-2,8-dimethyl-1,7-dioxaspiro[5.5]undecane and (E,E)-2-ethyl-8-methyl-1,7-dioxaspiro[5.5]undecane as components of a sex-attracting pheromone in B. frauenfeldi.


