Flupirtine maleateKV7 channel activator CAS# 75507-68-5 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 75507-68-5 | SDF | Download SDF |
| PubChem ID | 6435335 | Appearance | Powder |
| Formula | C19H21FN4O6 | M.Wt | 420.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | D 9998 | ||
| Solubility | DMSO : ≥ 40 mg/mL (95.15 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-[2-Amino-6-[[4-fluorophenyl)methy | ||
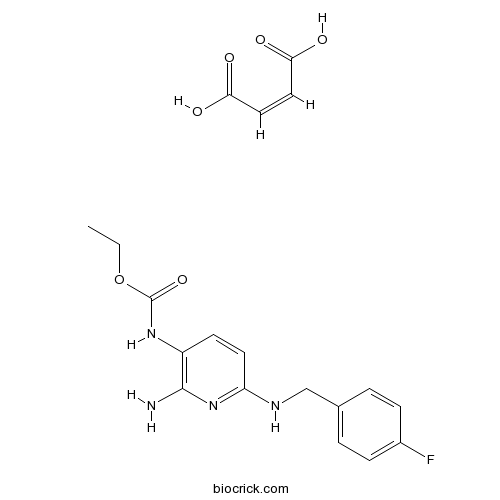
| SMILES | CCOC(=O)Nc1ccc(NCc2ccc(F)cc2)nc1N.OC(=O)C=C/C(O)=O | ||
| Standard InChIKey | DPYIXBFZUMCMJM-BTJKTKAUSA-N | ||
| Standard InChI | InChI=1S/C15H17FN4O2.C4H4O4/c1-2-22-15(21)19-12-7-8-13(20-14(12)17)18-9-10-3-5-11(16)6-4-10;5-3(6)1-2-4(7)8/h3-8H,2,9H2,1H3,(H,19,21)(H3,17,18,20);1-2H,(H,5,6)(H,7,8)/b;2-1- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-opioid analgesic with muscle relaxant properties. Activates KV7 potassium channels, indirectly antagonizes NMDA receptors and modulates GABAA receptors. Exhibits neuroprotective actions in a model of cerebral ischemia in mice and reduces apoptosis and necrosis induced by noxious stimuli. |

Flupirtine maleate Dilution Calculator

Flupirtine maleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3787 mL | 11.8937 mL | 23.7874 mL | 47.5749 mL | 59.4686 mL |
| 5 mM | 0.4757 mL | 2.3787 mL | 4.7575 mL | 9.515 mL | 11.8937 mL |
| 10 mM | 0.2379 mL | 1.1894 mL | 2.3787 mL | 4.7575 mL | 5.9469 mL |
| 50 mM | 0.0476 mL | 0.2379 mL | 0.4757 mL | 0.9515 mL | 1.1894 mL |
| 100 mM | 0.0238 mL | 0.1189 mL | 0.2379 mL | 0.4757 mL | 0.5947 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Flupirtine Maleate is Non-opioid analgesic with muscle relaxant properties. Activates K+ channels and indirectly antagonizes NMDA receptors.
- BI6727 (Volasertib)
Catalog No.:BCC3886
CAS No.:755038-65-4
- BI 2536
Catalog No.:BCC2081
CAS No.:755038-02-9
- Regorafenib
Catalog No.:BCC3646
CAS No.:755037-03-7
- N-Methylnuciferine
Catalog No.:BCN3971
CAS No.:754919-24-9
- CGP 37157
Catalog No.:BCC6943
CAS No.:75450-34-9
- Moxonidine
Catalog No.:BCC2142
CAS No.:75438-57-2
- EHT 1864
Catalog No.:BCC6075
CAS No.:754240-09-0
- Boc-Asp(OBzl)-OH
Catalog No.:BCC2608
CAS No.:7536-58-5
- Boc-Asn-OH
Catalog No.:BCC3071
CAS No.:7536-55-2
- Indacaterol Maleate
Catalog No.:BCC4358
CAS No.:753498-25-8
- Lovastatin
Catalog No.:BCN1060
CAS No.:75330-75-5
- H-Leucinol
Catalog No.:BCC2725
CAS No.:7533-40-6
- Cedrin
Catalog No.:BCN4748
CAS No.:75513-81-4
- Nilvadipine
Catalog No.:BCC3799
CAS No.:75530-68-6
- Moxonidine hydrochloride
Catalog No.:BCC5163
CAS No.:75536-04-8
- Dencichin
Catalog No.:BCN2555
CAS No.:7554-90-7
- Ingenol 3-Angelate
Catalog No.:BCN2961
CAS No.:75567-37-2
- 20-Deoxyingenol 3-angelate
Catalog No.:BCN6642
CAS No.:75567-38-3
- Sodium phosphate dibasic
Catalog No.:BCC7585
CAS No.:7558-79-4
- Sodium phosphate monobasic
Catalog No.:BCC8033
CAS No.:7558-80-7
- alpha-Tocopherolquinone
Catalog No.:BCN4305
CAS No.:7559-04-8
- Kaerophyllin
Catalog No.:BCN4304
CAS No.:75590-33-9
- Methyl 3,4,5-trimethoxycinnamate
Catalog No.:BCN4589
CAS No.:7560-49-8
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
Identification of Degradation Products and a Stability-Indicating RP-HPLC Method for the Determination of Flupirtine Maleate in Pharmaceutical Dosage Forms.[Pubmed:24959399]
Sci Pharm. 2013 Dec 9;82(2):281-93.
In this stability-indicating, reversed-phase high-performance liquid chromatographic method for flupiritine maleate, forced degradation has been employed and the formed degradants were separated on a C18 column with a 80:20% v/v mixture of methanol-water containing 0.2% (v/v) triethylamine; the pH was adjusted to 3.1. The flow rate was 1 mLmin(-1) and the photodiode array detection wavelength was 254 nm. Forced degradation of the drug was carried out under acidic, basic, thermal, photolytic, peroxide, and neutral conditions. Chromatographic peak purity data indicated no co-eluting peaks with the main peaks. This method resulted in the detection of seven degradation products (D1-D7). Among these, three major degradation products from acidic and basic hydrolysis were identified and characterized by (1)H-NMR, (13)C-NMR, and mass spectral data. The method was validated as per International Conference on Harmonization guidelines (Q2). The linearity of the method was in the concentration range of 20-120 mugmL(-1). The relative standard deviations for intra- and interday precision were below 1.5%. The specificity of the method is suitable for the stability-indicating assay.
Isolation, identification and characterization of novel process-related impurities in flupirtine maleate.[Pubmed:24333703]
J Pharm Biomed Anal. 2014 Mar;90:27-34.
Flupirtine maleate is a centrally acting, non-opioid, nonsteroidal antiinflammatory analgesic. During the manufacturing of Flupirtine maleate, two unknown impurities present in the laboratory batches in the range of 0.05-1.0% along with the known impurities in HPLC analysis. These unknown impurities were obtained from the enriched mother liquor by column chromatography. Based on the complete spectral analysis (MS, (1)H, (13)C, 2D NMR and IR) and knowledge of the synthetic scheme of Flupirtine maleate, these two new impurities were designated as diethyl 5-((4-fluorobenzyl)amino)-2-oxo-1H-imidazo[4,5-b]pyridine-1,3(2H)-dicarboxylate (impurity-I) and diethyl(6-((4-fluorobenzyl)amino)pyridine-2,3-diyl)dicarbamate (impurity-II). Impurity isolation, identification, structure elucidation and the formation of impurities were also discussed. Preparation and structure elucidation of impurity-III were also first reported in this paper.
Efficacy and safety of flupirtine maleate and tramadol hydrochloride in postoperative pain management--a prospective randomised double blinded study.[Pubmed:23029946]
J Indian Med Assoc. 2012 Mar;110(3):158-60.
The study was conducted to evaluate the efficacy and safety of Flupirtine maleate 100 mg thrice daily compared to tramadol hydrochloride 50 mg thrice daily as postoperative pain management for 5 days. A total of 113 postoperative patients were recruited for the study. Those who met the inclusion criteria (n = 104) were randomised into two treatment groups. One of the groups received Flupirtine maleate and the other tramadol hydrochloride both orally. The pain intensity was assessed by visual analogue scale. Patients were informed to report any adverse effect encountered during the study period. The overall effect of the drug (global assessment of the study medication) on pain and side-effects was assessed by the patients at the end of the trial on a categorical scale. There was significant reduction in pain score (p < 0.001) in the flupirtine group with almost equal efficacy to that of tramadol group but the incidence of adverse effects were much less (7.4%) and didn't need discontinuation of the study. All drugs were assessed as good. Therefore it can be concluded that oral flupirtine can deliver the same analgesic efficacy as oral tramadol for postoperative pain relief, which might be beneficial for avoiding the adverse effects ofopioids and non-steroidal anti-inflammatory drug therapy.
Comparison of analgesic efficacy of flupirtine maleate and ibuprofen in gynaecological ambulatory surgeries: A randomized controlled trial.[Pubmed:26257413]
Indian J Anaesth. 2015 Jul;59(7):411-5.
BACKGROUND AND AIMS: Flupirtine maleate is a centrally acting, non-opioid analgesic with unique muscle relaxant properties as compared to common analgesics. The aim of this study was to compare post-operative analgesic efficacy of Flupirtine maleate and ibuprofen in patients undergoing gynaecological ambulatory surgeries. METHODS: This prospective, randomised controlled study was conducted in 60 women of American Society of Anesthesiologists physical status I/II, 18-70 years of age and scheduled to undergo gynaecological ambulatory surgeries. The participants were randomised to receive either 100 mg oral Flupirtine maleate (group flupirtine, n = 30) or 800 mg oral ibuprofen (group ibuprofen, n = 30), 1 h prior to surgery and then every 8 h for 48 h. Verbal Numerical Rating Scale (VNRS) on movement was assessed at 0, 2, 4, 6 and 8 h following surgery. Following discharge from hospital, the patients were interviewed telephonically at 12, 24 and 48 h post-operatively. VNRS was statistically analysed using Mann-Whitney test. RESULTS: VNRS on movement was statistically reduced at 2 h after surgery (P = 0.04) in group flupirtine as compared to group ibuprofen. The analgesic efficacy was similar in both the groups at 4, 6, 8, 12, 24 and 48 h after surgery. The satisfaction scores at 24 and 48 h post-operatively were superior in group flupirtine as compared to group ibuprofen (P < 0.001). CONCLUSION: Analgesic efficacy of Flupirtine maleate was comparable with ibuprofen in patients in ambulatory gynaecological patients up to 48 h postoperatively with superior satisfaction scores.
Concomitant facilitation of GABAA receptors and KV7 channels by the non-opioid analgesic flupirtine.[Pubmed:22188423]
Br J Pharmacol. 2012 Jul;166(5):1631-42.
BACKGROUND AND PURPOSE: Flupirtine is a non-opioid analgesic that has been in clinical use for more than 20 years. It is characterized as a selective neuronal potassium channel opener (SNEPCO). Nevertheless, its mechanisms of action remain controversial and are the purpose of this study. EXPERIMENTAL APPROACH: Effects of flupirtine on native and recombinant voltage- and ligand-gated ion channels were explored in patch-clamp experiments using the following experimental systems: recombinant K(IR)3 and K(V)7 channels and alpha3beta4 nicotinic acetylcholine receptors expressed in tsA 201 cells; native voltage-gated Na(+), Ca(2+), inward rectifier K(+), K(V)7 K(+), and TRPV1 channels, as well as GABA(A), glycine, and ionotropic glutamate receptors expressed in rat dorsal root ganglion, dorsal horn and hippocampal neurons. KEY RESULTS: Therapeutic flupirtine concentrations (
Molecular expression and pharmacological identification of a role for K(v)7 channels in murine vascular reactivity.[Pubmed:17519950]
Br J Pharmacol. 2007 Jul;151(6):758-70.
BACKGROUND AND PURPOSE: This study represents a novel characterisation of KCNQ-encoded potassium channels in the vasculature using a variety of pharmacological and molecular tools to determine their role in contractility. EXPERIMENTAL APPROACH: Reverse transcriptase polymerase chain reaction (RT-PCR) experiments were undertaken on RNA isolated from mouse aorta, carotid artery, femoral artery and mesenteric artery using primers specific for all known KCNQ genes. RNA isolated from mouse heart and brain were used as positive controls. Pharmacological experiments were undertaken on segments from the same blood vessels to determine channel functionality. Immunocytochemical experiments were performed on isolated myocytes from thoracic aorta. KEY RESULTS: All blood vessels expressed KCNQ1, 4 and 5 with hitherto 'neuronal' KCNQ4 being, surprisingly, the most abundant. The correlated proteins K(v)7.1, K(v)7.4 and K(v)7.5 were identified in the cell membranes of aortic myocytes by immunocytochemistry. Application of three compounds known to activate K(v)7 channels, retigabine (2 -20 microM), flupirtine (20 microM) and meclofenamic acid (20 microM), relaxed vessels precontracted by phenylephrine or 1 mM 4-aminopyridine but had no effect on contractions produced by 60 mM KCl or the K(v)7 channel blocker XE991 (10 microM). All vessels tested contracted upon application of the K(v)7 channel blockers XE991 and linopirdine (0.1-10 microM). CONCLUSIONS AND IMPLICATIONS: Murine blood vessels exhibit a distinctive KCNQ expression profile with 'neuronal' KCNQ4 dominating. The ion channels encoded by KCNQ genes have a crucial role in defining vascular reactivity as K(v)7 channel blockers produced marked contractions whereas K(v)7 channel activators were effective vasorelaxants.
The potassium channel modulator flupirtine shifts the frequency-response function of hippocampal synapses to favour LTD in mice.[Pubmed:15488320]
Neurosci Lett. 2004 Nov 11;370(2-3):186-90.
Flupirtine is a centrally acting nonopioid analgesic with muscle-relaxant properties. Flupirtine has been found to activate inwardly rectifying potassium conductances and hence to indirectly inhibit the activation of NMDA receptors. NMDA receptor activation is crucial for the induction of long-term potentiation (LTP) of synaptic transmission, which is considered as cellular correlate of learning and memory and of central sensitization in chronic pain states. Although flupirtine has been widely used for the management of pain, its effects on synaptic plasticity have not yet been investigated. We, therefore, performed extracellular and whole-cell patch-clamp recordings in hippocampal slices of mice to examine the effects of flupirtine on synaptic plasticity and neuronal membrane properties. Excitatory postsynaptic potentials (EPSPs) in the CA1 region were evoked alternately by stimulating two independent Schaffer collateral-commissural inputs. LTP and long-term depression (LTD) were induced by different stimulation paradigms (100 Hz, 10 Hz, 5 Hz, and 1 Hz). Flupirtine (30 microM) diminished the degree of LTP and enhanced LTD. This effect is most likely due to the hyperpolarization of CA1 pyramidal neurons and the reduction of their input resistance found after application of flupirtine. The observed effects on synaptic strength could underly the beneficial effects of flupirtine on different types of chronic pain.
Flupirtine, a nonopioid centrally acting analgesic, acts as an NMDA antagonist.[Pubmed:9510072]
Gen Pharmacol. 1998 Mar;30(3):255-63.
1. Flupirtine (Katadolon) is a member of a class of triaminopyridines and is used as a nonopioid analgesic agent with muscle relaxant properties. 2. In situ experiments have revealed that flupirtine protects against ischemic-induced insults to the retina and brain. 3. Data derived from in vitro and in vivo studies suggest that flupirtine functions as a weak N-methyl-D-aspartate (NMDA) antagonist with little evidence that it acts on AMPA-kainate type glutamate receptors. 4. No evidence could be found from binding studies to suggest that flupirtine has an affinity for any of the characterized binding sites associated with the NMDA receptor. 5. Studies on cultured cortical neurons show that the NMDA-induced influx of 45Ca2+ is more readily decreased by flupirtine when a reducing agent (dithiothreitol) is present. However, when N'-ethylmaleimide, which is thought to alkylate the NMDA receptor redox site, is present, no obvious effect on the NMDA-induced influx of 45Ca2+ is produced by flupirtine. 6. Flupirtine is also known to counteract the production of reactive oxygen species caused by ascorbate/iron as well as to prevent apoptosis in cells lacking NMDA receptors induced by oxidative stress. 7. To explain all the experimental data, it is suggested that flupirtine affects the redox state/pH/electrons in the cell. The specific way by which flupirtine antagonizes the NMDA receptor might be by an action on the known redox site of the receptor.


