EtomidateGeneral anesthetic with GABA modulatory and GABA-mimetic actions CAS# 33125-97-2 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
- Flumazenil
Catalog No.:BCC1259
CAS No.:78755-81-4
Quality Control & MSDS
Number of papers citing our products

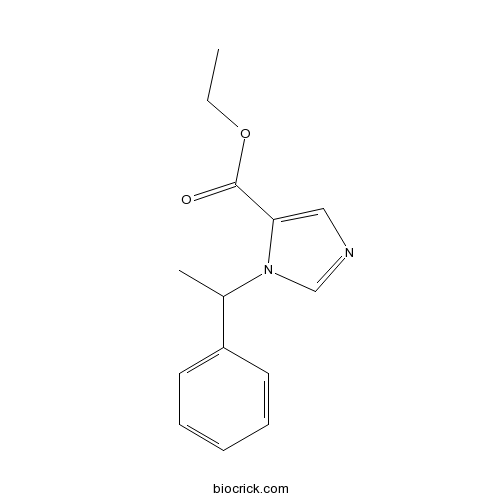
Chemical structure

3D structure
| Cas No. | 33125-97-2 | SDF | Download SDF |
| PubChem ID | 36339 | Appearance | Powder |
| Formula | C14H16N2O2 | M.Wt | 244.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | R 16659 | ||
| Solubility | DMSO : ≥ 100 mg/mL (409.35 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | ethyl 3-(1-phenylethyl)imidazole-4-carboxylate | ||
| SMILES | CCOC(=O)C1=CN=CN1C(C)C2=CC=CC=C2 | ||
| Standard InChIKey | NPUKDXXFDDZOKR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H16N2O2/c1-3-18-14(17)13-9-15-10-16(13)11(2)12-7-5-4-6-8-12/h4-11H,3H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | General anesthetic with GABA modulatory and GABA-mimetic actions; selectively interacts with β2- and β3-subunit containing GABAA receptors. Short acting and potent hypnotic, with low toxicity. |

Etomidate Dilution Calculator

Etomidate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0935 mL | 20.4675 mL | 40.935 mL | 81.8699 mL | 102.3374 mL |
| 5 mM | 0.8187 mL | 4.0935 mL | 8.187 mL | 16.374 mL | 20.4675 mL |
| 10 mM | 0.4093 mL | 2.0467 mL | 4.0935 mL | 8.187 mL | 10.2337 mL |
| 50 mM | 0.0819 mL | 0.4093 mL | 0.8187 mL | 1.6374 mL | 2.0467 mL |
| 100 mM | 0.0409 mL | 0.2047 mL | 0.4093 mL | 0.8187 mL | 1.0234 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
General anesthetic with GABA modulatory and GABA-mimetic actions; selectively interacts with β2- and β3-subunit containing GABAA receptors. Short acting and potent hypnotic, with low toxicity.
- Boc-D-Phg-OH
Catalog No.:BCC3315
CAS No.:33125-05-2
- LG 101506
Catalog No.:BCC7696
CAS No.:331248-11-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Stephavanine
Catalog No.:BCN5253
CAS No.:33116-33-5
- PT 1
Catalog No.:BCC7846
CAS No.:331002-70-1
- IQ 1
Catalog No.:BCC7965
CAS No.:331001-62-8
- Caffeic acid
Catalog No.:BCN5979
CAS No.:331-39-5
- AS 1269574
Catalog No.:BCC7878
CAS No.:330981-72-1
- Betrixaban
Catalog No.:BCC5118
CAS No.:330942-05-7
- Amitraz
Catalog No.:BCC8816
CAS No.:33089-61-1
- MRT 10
Catalog No.:BCC7950
CAS No.:330829-30-6
- PCI 29732
Catalog No.:BCC4100
CAS No.:330786-25-9
- trans-2,3,4-Trimethoxycinnamic acid
Catalog No.:BCN5035
CAS No.:33130-03-9
- CFM 4
Catalog No.:BCC8017
CAS No.:331458-02-7
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- DMPO
Catalog No.:BCC7684
CAS No.:3317-61-1
- Bisdemethoxycurcumin
Catalog No.:BCN5975
CAS No.:33171-05-0
- 10-Demethoxy-10-(diethylamino)colchicine
Catalog No.:BCC8164
CAS No.:6962-03-4
- ZM 447439
Catalog No.:BCC2169
CAS No.:331771-20-1
- 2-(N,N-Dimethylamino)acetophenone
Catalog No.:BCN1747
CAS No.:3319-03-7
- 2',4'-Dihydroxy-7-methoxy-8-prenylflavan
Catalog No.:BCN6844
CAS No.:331954-16-6
- I2906
Catalog No.:BCC1637
CAS No.:331963-29-2
- Nτ-Methyl-His-OH
Catalog No.:BCC2957
CAS No.:332-80-9
- Telatinib (BAY 57-9352)
Catalog No.:BCC3879
CAS No.:332012-40-5
Effects of anesthesia using propofol and etomidate on T lymphocyte subpopulation of infectious shock patients in perioperative period.[Pubmed:28337880]
J Biol Regul Homeost Agents. 2017 Jan-Mar;31(1):119-123.
Infectious or septic shock is induced when a toxic microorganism invades blood circulation in the human body. Emergency operation is an effective method for treating infectious shock in the early stages although the use of anesthesia is more complex due to the internal disorders caused by the disease. This study explored the effects of propofol and Etomidate anesthesia on the cellular immune function (T lymphocyte subpopulation) of infectious shock patients, aiming to provide a basis for the selection of the proper anesthetic method. One hundred and twenty patients with infectious shock were selected and randomly divided into an observation and a control group. The control group were narcotized using propofol, while the observation group were narcotized using Etomidate. The effects on the immune functions of patients and drug-related adverse reactions were compared between the two groups. Results demonstrated that the levels of CD3+ and CD4+ of the two groups were similar before anesthesia and the differences had no statistical significance (P 0.05). After anesthesia, the levels of both groups showed a tendency to decrease and the levels of CD3+ and CD4+ of the observation group were much higher than those of the control group in the different periods. The differences were statistically significant (P less than 0.05); the differences of CD8+ level and CD4+/CD8+ between the two groups had no statistical significance before anesthesia (P0.05); after anesthesia, CD8+ level and CD4+/CD8+ of the observation group were all much higher than those of the control group in the different periods and the differences had statistical significance (P less than 0.05). Therefore, the conclusion is that Etomidate anesthesia has little influence on the immune functions of infectious shock patients in perioperative period and the incidence of adverse reaction is low, hence, worth clinical promotion.
Continuous-Infusion Etomidate in a Patient Receiving Extracorporeal Membrane Oxygenation.[Pubmed:28337083]
J Pediatr Pharmacol Ther. 2017 Jan-Feb;22(1):65-68.
We describe a 16-year-old, 65-kg male deployed on extracorporeal membrane oxygenation (ECMO) for refractory respiratory failure secondary to ingestion of multiple substances. During his ECMO course, standard sedative and analgesic strategies failed and alternative medications were used. The patient received various dosages of fentanyl, morphine, hydromorphone, clonidine patches, dexmedetomidine, lorazepam, methadone, pentobarbital, olanzapine, and propofol. Despite administration of multiple agents, on day 29 of ECMO the patient experienced elevated blood pressures due to agitation, and continuous infusion Etomidate was started. At the time of Etomidate initiation, the osmolar gap was 8 mOsm/kg. During Etomidate therapy, the blood pressure remained normal, sedative agents were slowly weaned, and the patient required few PRN medications. On day 6 of Etomidate, the osmolar gap increased to 127 mOsm/kg and Etomidate was discontinued. Continuous-infusion ketamine was started, but the blood pressure was not controlled. Metabolic acidosis is a known side effect of Etomidate due to inclusion of propylene glycol as a pharmaceutical solvent in the formulation. Despite high-dose Etomidate (20 mcg/kg/min) for approximately 6 days, our patient did not experience metabolic acidosis. Absence of this adverse effect caused us to question the role of the ECMO circuit. To our knowledge, this is the first report of the use of continuous-infusion Etomidate during ECMO. Etomidate infusion could be considered in difficult-to-manage patients after other alternatives have failed.
Parecoxib sodium pretreatment reduces myoclonus after etomidate: A prospective, double-blind, randomized clinical trial.[Pubmed:28291508]
Int J Clin Pharmacol Ther. 2017 Jul;55(7):601-605.
OBJECTIVE: Myoclonus induced by Etomidate during induction of general anesthesia is a common phenomenon. This prospective, randomized, saline-controlled clinical study was performed to evaluate the effect of parecoxib sodium pretreatment on the incidence and severity of Etomidate-induced myoclonus. METHODS: 60 patients, American Society of Anesthesiologists (ASA) physical status I or II, aged 20 to 60 years, who were scheduled to undergo elective laparoscopic cholecystectomy under general anesthesia, were allocated randomly into one of two groups to receive parecoxib sodium 40 mg intravenous (group P, n = 30) or the same volume of saline (group S, n = 30) 30 minutes before administration of Etomidate (0.3 mg/kg). Myoclonus was assessed on a scale of 0 - 3. Postoperative side effects were recorded. RESULTS: The two groups were comparable with regard to baseline characteristics. The incidence of myoclonus was significantly lower in the parecoxib sodium group (11/30; 37%) than in the saline group (21/30; 70%) (p < 0.05). The severity of myoclonic movements was also significantly reduced by parecoxib sodium (p < 0.05). There were no significant differences between the two groups with respect to postoperative side effects. CONCLUSIONS: Pretreatment with intravenous injection of parecoxib sodium 40 mg significantly reduced the incidence and severity of Etomidate-induced myoclonus without significant side effects..
Etomidate versus propofol sedation for complex upper endoscopic procedures: a prospective double-blinded randomized controlled trial.[Pubmed:28284883]
Gastrointest Endosc. 2017 Sep;86(3):452-461.
BACKGROUND AND AIMS: Although a growing body of evidence demonstrates that propofol-induced deep sedation can be effective and performed safely, cardiopulmonary adverse events have been observed frequently. Etomidate is a new emerging drug that provides hemodynamic and respiratory stability, even in high-risk patient groups. The objective of this study was to compare safety and efficacy profiles of Etomidate and propofol for endoscopic sedation. METHODS: A total of 128 patients undergoing EUS were randomized to receive either Etomidate or propofol blinded administered by a registered nurse. The primary outcome was the proportion of patients with any cardiopulmonary adverse events. RESULTS: Overall cardiopulmonary adverse events were identified in 22 patients (34.38%) of the Etomidate group and 33 patients (51.56%) of the propofol group, without significant difference (P = .074). However, the incidence of oxygen desaturation (4/64 [6.25%] vs 20/64 [31.25%]; P =.001) and respiratory depression (5/64 [7.81%] vs 21/64 [32.81%]; P =.001) was significantly lower in the Etomidate group than in the propofol group. The frequency of myoclonus was significantly higher in the Etomidate group (22/64 [34.37%]) compared with the propofol group (8/64 [12.50%]) (P =.012). Repeated measure analysis of variance revealed significant effects of sedation group and time on systolic blood pressure (Etomidate group greater than propofol group). Physician satisfaction was greater in the Etomidate group than in the propofol group. CONCLUSIONS: Etomidate administration resulted in fewer respiratory depression events and had a better sedative efficacy than propofol; however, it was more frequently associated with myoclonus and increased blood pressure during endoscopic procedures. (Clinical trial registration number: KCT0001701.).
Distinct structural requirements for the direct and indirect actions of the anaesthetic etomidate at GABA(A) receptors.[Pubmed:10049144]
Toxicol Lett. 1998 Nov 23;100-101:209-15.
1. The intravenous anaesthetic Etomidate augments GABA-gated chloride currents (indirect action) and, at higher concentrations, evokes chloride currents in the absence of GABA (direct action). 2. In order to identify amino acid residues essential for these actions, site directed mutagenesis was performed on the beta3 subunit. 3. Mutation of an asparagine to a serine residue at position 290 dramatically reduced both Etomidate-induced chloride currents and its ability to enhance [3H]flunitrazepam binding in HEK293 cells expressing alpha1beta3gamma2 recombinant GABA(A) receptors. 4. In contrast, the indirect effect of Etomidate was retained, though its potency was reduced. 5. These findings indicate that there are distinct requirements for these dual actions of Etomidate at GABA(A) receptors.
The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid.[Pubmed:9380754]
Proc Natl Acad Sci U S A. 1997 Sep 30;94(20):11031-6.
The gamma-aminobutyric acid type A (GABAA) receptor is a transmitter-gated ion channel mediating the majority of fast inhibitory synaptic transmission within the brain. The receptor is a pentameric assembly of subunits drawn from multiple classes (alpha1-6, beta1-3, gamma1-3, delta1, and epsilon1). Positive allosteric modulation of GABAA receptor activity by general anesthetics represents one logical mechanism for central nervous system depression. The ability of the intravenous general anesthetic Etomidate to modulate and activate GABAA receptors is uniquely dependent upon the beta subunit subtype present within the receptor. Receptors containing beta2- or beta3-, but not beta1 subunits, are highly sensitive to the agent. Here, chimeric beta1/beta2 subunits coexpressed in Xenopus laevis oocytes with human alpha6 and gamma2 subunits identified a region distal to the extracellular N-terminal domain as a determinant of the selectivity of Etomidate. The mutation of an amino acid (Asn-289) present within the channel domain of the beta3 subunit to Ser (the homologous residue in beta1), strongly suppressed the GABA-modulatory and GABA-mimetic effects of Etomidate. The replacement of the beta1 subunit Ser-290 by Asn produced the converse effect. When applied intracellularly to mouse L(tk-) cells stably expressing the alpha6beta3gamma2 subunit combination, Etomidate was inert. Hence, the effects of a clinically utilized general anesthetic upon a physiologically relevant target protein are dramatically influenced by a single amino acid. Together with the lack of effect of intracellular Etomidate, the data argue against a unitary, lipid-based theory of anesthesia.
Etomidate: a new intravenous anesthetic induction agent.[Pubmed:6359080]
Pharmacotherapy. 1983 Sep-Oct;3(5):251-8.
Currently available anesthetic induction agents provide adequate hypnosis but are not ideal, particularly in the high risk patient (ASA class III-V), because most cause myocardial and/or respiratory depression and some have other important side effects. Etomidate was recently marketed as an intravenous anesthetic induction agent. It is a non-barbiturate hypnotic without analgesic properties that has less cardiovascular and respiratory depressant actions than sodium thiopental, even in patients with minimal cardiovascular reserve. Laboratory studies indicate that Etomidate is approximately 25 times more potent and has a therapeutic index six times greater than sodium thiopental. In contrast to most other induction agents, Etomidate does not cause histamine release. Furthermore, tolerance does not occur with repeated administration. Etomidate's rapid distribution half life (t 1/2 alpha = 2.81 +/- 1.64 min), short elimination half life 1/2 beta = 3.88 +/- 1.11 hr) and rapid clearance (954 +/- 178 ml/min) explain its rapid onset and short duration of action. The compound produces electroencephalographic changes and effects on cerebral blood flow, metabolism and intracranial pressure that are similar to sodium thiopental, suggesting that it may have a place in neurosurgery and as a "brain protective" agent in patients at risk of a brain hypoxic insult. Etomidate did not affect hepatorenal and hematologic function after repeated injections in animal toxicology studies, but few investigations addressing its effects on hepatic, renal, and neuromuscular function in man have been accomplished. The most noticeable side effects of Etomidate include myoclonia, pain on injection and postoperative nausea and vomiting.(ABSTRACT TRUNCATED AT 250 WORDS)


