Caffeic acidCAS# 331-39-5 |
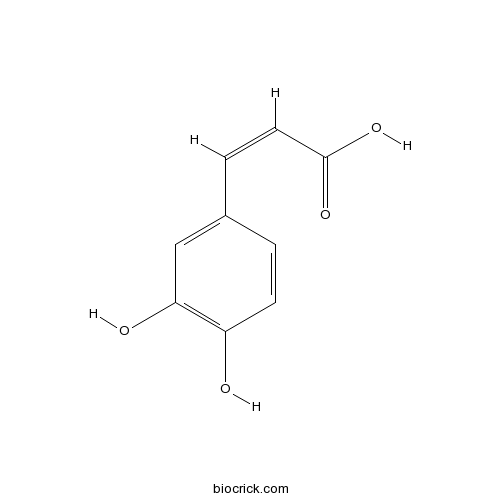
- Trans-caffeic acid
Catalog No.:BCN3462
CAS No.:501-16-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 331-39-5 | SDF | Download SDF |
| PubChem ID | 1549111 | Appearance | Powder |
| Formula | C9H8O4 | M.Wt | 180.15 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 106.7 mg/mL (592.25 mM; Need ultrasonic and warming) | ||
| Chemical Name | (Z)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid | ||
| SMILES | C1=CC(=C(C=C1C=CC(=O)O)O)O | ||
| Standard InChIKey | QAIPRVGONGVQAS-RQOWECAXSA-N | ||
| Standard InChI | InChI=1S/C9H8O4/c10-7-3-1-6(5-8(7)11)2-4-9(12)13/h1-5,10-11H,(H,12,13)/b4-2- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Caffeic acid has antidiabetic, antioxidant, anticarcinogenic, and anti-inflammatory activities, it can suppress ultraviolet B(UVB)-induced COX-2 expression by blocking Fyn kinase activity, inhibits HBV-DNA replication as well as HBsAg production, also reduces serum DHBV level in DHBV-infected duckling model. Caffeic acid may be used as designed novel therapeutic drugs for Parkinson's disease by inhibiting α-synuclein fibrillation. |
| Targets | Estrogen receptor | IGF-1R | HBV | AP-1 | COX | MAPK | NF-kB | NO | NOS | IL Receptor | Progestogen receptor |
| In vitro | Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro.[Pubmed: 19463857 ]Antiviral Res. 2009 Aug;83(2):186-90.Chlorogenic acid and its related compounds are abundant plant polyphenols that have a diverse antiviral activity.
|
| In vivo | Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice.[Pubmed: 16644902 ]J Pharmacol Exp Ther. 2006 Aug;318(2):476-83This study investigated the blood glucose-lowering effect and antioxidant capacity of Caffeic acid in C57BL/KsJ-db/db mice.
Caffeic acid derivatives: in vitro and in vivo anti-inflammatory properties.[Pubmed: 15621702]Free Radic Res. 2004 Nov;38(11):1241-53.Caffeic acid and some of its derivatives such as Caffeic acid phenetyl ester (CAPE) and octyl caffeate are potent antioxidants which present important anti-inflammatory actions. |
| Kinase Assay | Anti-fibrillation potency of caffeic acid against an antidepressant induced fibrillogenesis of human α-synuclein: Implications for Parkinson's disease.[Pubmed: 25461276]Biochimie. 2015 Jan;108:178-85.Alpha synuclein is a 14 kDa intrinsically disordered, presynaptic protein whose fibrillation is a critical step in the pathogenesis of Parkinson's disease (PD). A structural investigation of the effect of escitalopram (a selective serotonin reuptake inhibitor) on α-synuclein was performed using ANS and ThT assays, CD, turbidity and Rayleigh scattering measurements as well as atomic force and transmission electron microscopy. Analysing the mechanism of α-synuclein fibril formation, helped us in elucidating the passage of an intermediate at 75 μM concentration of escitalopram. Fibrils of α-synuclein were obtained at 100 μM concentration of escitalopram. Inhibition of α-synuclein fibrillation was brought about by a polyphenolic acid known as Caffeic acid which acted in a concentration dependent manner ranging from 10 to 60 μM. Maximum inhibition was achieved at a concentration of 60 μM. Fibrillation of α-synuclein in presence of escitalopram gives us clue for the negative effects of antidepressant. Inhibitory activity of Caffeic acid against α-synuclein fibrillation may guide us in designing novel therapeutic drugs for PD. |
| Cell Research | Caffeine and caffeic acid inhibit growth and modify estrogen receptor (ER) and insulin-like growth factor I receptor (IGF-IR) levels in human breast cancer.[Pubmed: 25691730]Clin Cancer Res. 2015 Feb 17.Epidemiologic studies indicate that dietary factors, such as coffee, may influence breast cancer and modulate hormone receptor status. The purpose of this translational study was to investigate how coffee may affect breast cancer growth in relation to estrogen receptor-α (ER) status.
|
| Animal Research | Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression[Pubmed: 19073879]Carcinogenesis. 2009 February; 30(2): 321–30.Caffeic acid (3,4-dihydroxycinnamic acid) is a well-known phenolic phytochemical present in many foods, including coffee. Recent studies suggested that Caffeic acid exerts anticarcinogenic effects, but little is known about the underlying molecular mechanisms and specific target proteins.
|

Caffeic acid Dilution Calculator

Caffeic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.5509 mL | 27.7546 mL | 55.5093 mL | 111.0186 mL | 138.7732 mL |
| 5 mM | 1.1102 mL | 5.5509 mL | 11.1019 mL | 22.2037 mL | 27.7546 mL |
| 10 mM | 0.5551 mL | 2.7755 mL | 5.5509 mL | 11.1019 mL | 13.8773 mL |
| 50 mM | 0.111 mL | 0.5551 mL | 1.1102 mL | 2.2204 mL | 2.7755 mL |
| 100 mM | 0.0555 mL | 0.2775 mL | 0.5551 mL | 1.1102 mL | 1.3877 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AS 1269574
Catalog No.:BCC7878
CAS No.:330981-72-1
- Betrixaban
Catalog No.:BCC5118
CAS No.:330942-05-7
- Amitraz
Catalog No.:BCC8816
CAS No.:33089-61-1
- MRT 10
Catalog No.:BCC7950
CAS No.:330829-30-6
- PCI 29732
Catalog No.:BCC4100
CAS No.:330786-25-9
- Avanafil
Catalog No.:BCC2288
CAS No.:330784-47-9
- Paclitaxel
Catalog No.:BCN4650
CAS No.:33069-62-4
- KH 7
Catalog No.:BCC7787
CAS No.:330676-02-3
- HCTU
Catalog No.:BCC2818
CAS No.:330645-87-9
- TCTU
Catalog No.:BCC2689
CAS No.:330641-16-2
- Peramivir
Catalog No.:BCC1846
CAS No.:330600-85-6
- H-Orn(Z)-OH
Catalog No.:BCC3003
CAS No.:3304-51-6
- IQ 1
Catalog No.:BCC7965
CAS No.:331001-62-8
- PT 1
Catalog No.:BCC7846
CAS No.:331002-70-1
- Stephavanine
Catalog No.:BCN5253
CAS No.:33116-33-5
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- LG 101506
Catalog No.:BCC7696
CAS No.:331248-11-4
- Boc-D-Phg-OH
Catalog No.:BCC3315
CAS No.:33125-05-2
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- trans-2,3,4-Trimethoxycinnamic acid
Catalog No.:BCN5035
CAS No.:33130-03-9
- CFM 4
Catalog No.:BCC8017
CAS No.:331458-02-7
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- DMPO
Catalog No.:BCC7684
CAS No.:3317-61-1
- Bisdemethoxycurcumin
Catalog No.:BCN5975
CAS No.:33171-05-0
Caffeine and Caffeic Acid Inhibit Growth and Modify Estrogen Receptor and Insulin-like Growth Factor I Receptor Levels in Human Breast Cancer.[Pubmed:25691730]
Clin Cancer Res. 2015 Apr 15;21(8):1877-87.
PURPOSE: Epidemiologic studies indicate that dietary factors, such as coffee, may influence breast cancer and modulate hormone receptor status. The purpose of this translational study was to investigate how coffee may affect breast cancer growth in relation to estrogen receptor-alpha (ER) status. EXPERIMENTAL DESIGN: The influence of coffee consumption on patient and tumor characteristics and disease-free survival was assessed in a population-based cohort of 1,090 patients with invasive primary breast cancer in Sweden. Cellular and molecular effects by the coffee constituents caffeine and Caffeic acid were evaluated in ER(+) (MCF-7) and ER(-) (MDA-MB-231) breast cancer cells. RESULTS: Moderate (2-4 cups/day) to high (>/=5 cups/day) coffee intake was associated with smaller invasive primary tumors (Ptrend = 0.013) and lower proportion of ER(+) tumors (Ptrend = 0.018), compared with patients with low consumption (Caffeic acid suppressed the growth of ER(+) (P Caffeic acid against both ER(+) and ER(-) breast cancer that may sensitize tumor cells to tamoxifen and reduce breast cancer growth.
Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro.[Pubmed:19463857]
Antiviral Res. 2009 Aug;83(2):186-90.
Chlorogenic acid and its related compounds are abundant plant polyphenols that have a diverse antiviral activity. In this study, HepG2.2.15 cells and duck hepatitis B virus infection model were used as in vitro and in vivo models to evaluate their anti-HBV activity. In the cell model, all the three compounds inhibited HBV-DNA replication as well as HBsAg production. Chlorogenic acid and Caffeic acid also reduced serum DHBV level in DHBV-infected duckling model. Moreover, the anti-HBV activity of crude extracts of coffee beans, which have a high content of chlorogenic acid, was studied. Both the extracts of regular coffee and that of decaffeinated coffee showed inhibitory effect on HBV replication.
Caffeic acid derivatives: in vitro and in vivo anti-inflammatory properties.[Pubmed:15621702]
Free Radic Res. 2004 Nov;38(11):1241-53.
Caffeic acid and some of its derivatives such as Caffeic acid phenetyl ester (CAPE) and octyl caffeate are potent antioxidants which present important anti-inflammatory actions. The present study assessed the in vitro and in vivo effects of five Caffeic acid derivatives (Caffeic acid methyl, ethyl, butyl, octyl and benzyl esters) and compared their actions to those of CAPE. In the model of LPS-induced nitric oxide (NO) production in RAW 264.7 macrophages, the pre-incubation of all derivatives inhibited nitrite accumulation on the supernatant of stimulated cells, with mean IC50 (microM) values of 21.0, 12.0, 8.4, 2.4, 10.7 and 4.80 for methyl, ethyl, butyl, octyl, benzyl and CAPE, respectively. The effects of Caffeic acid derivatives seem to be related to the scavenging of NO, as the compounds prevented SNAP-derived nitrite accumulation and decreased iNOS expression. In addition, butyl, octyl and CAPE derivatives significantly inhibited LPS-induced iNOS expression in RAW 264.7 macrophages. Extending the in vitro results, we showed that the pre-treatment of mice with butyl, octyl and CAPE derivatives inhibited carrageenan-induced paw edema and prevented the increase in IL-1beta levels in the mouse paw by 30, 24 and 36%, respectively. Butyl, octyl and CAPE derivatives also prevented carrageenan-induced neutrophil influx in the mouse paw by 28, 49 and 31%, respectively. Present results confirm and extend literature data, showing that Caffeic acid derivatives exert in vitro and in vivo anti-inflammatory actions, being their actions mediated, at least in part by the scavenging of NO and their ability to modulate iNOS expression and probably that of other inflammatory mediators.
Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression.[Pubmed:19073879]
Carcinogenesis. 2009 Feb;30(2):321-30.
Caffeic acid (3,4-dihydroxycinnamic acid) is a well-known phenolic phytochemical present in many foods, including coffee. Recent studies suggested that Caffeic acid exerts anticarcinogenic effects, but little is known about the underlying molecular mechanisms and specific target proteins. In this study, we found that Fyn, one of the members of the non-receptor protein tyrosine kinase family, was required for ultraviolet (UV) B-induced cyclooxygenase-2 (COX-2) expression, and Caffeic acid suppressed UVB-induced skin carcinogenesis by directly inhibiting Fyn kinase activity. Caffeic acid more effectively suppressed UVB-induced COX-2 expression and subsequent prostaglandin E(2) production in JB6 P+ mouse skin epidermal (JB6 P+) cells compared with chlorogenic acid (5-O-caffeoylquinic acid), an ester of Caffeic acid with quinic acid. Data also revealed that Caffeic acid more effectively induced the downregulation of COX-2 expression at the transcriptional level mediated through the inhibition of activator protein-1 (AP-1) and nuclear factor-kappaB transcription activity compared with chlorogenic acid. Fyn kinase activity was suppressed more effectively by Caffeic acid than by chlorogenic acid, and downstream mitogen-activated protein kinases (MAPKs) were subsequently blocked. Pharmacological Fyn kinase inhibitor (3-(4-chlorophenyl)1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine and leflunomide) data also revealed that Fyn is involved in UVB-induced COX-2 expression mediated through the phosphorylation of MAPKs in JB6 P+ cells. Pull-down assays revealed that Caffeic acid directly bound with Fyn and non-competitively with adenosine triphosphate. In vivo data from mouse skin also supported the idea that Caffeic acid suppressed UVB-induced COX-2 expression by blocking Fyn kinase activity. These results suggested that this compound could act as a potent chemopreventive agent against skin cancer.
Anti-fibrillation potency of caffeic acid against an antidepressant induced fibrillogenesis of human alpha-synuclein: Implications for Parkinson's disease.[Pubmed:25461276]
Biochimie. 2015 Jan;108:178-85.
Alpha synuclein is a 14 kDa intrinsically disordered, presynaptic protein whose fibrillation is a critical step in the pathogenesis of Parkinson's disease (PD). A structural investigation of the effect of escitalopram (a selective serotonin reuptake inhibitor) on alpha-synuclein was performed using ANS and ThT assays, CD, turbidity and Rayleigh scattering measurements as well as atomic force and transmission electron microscopy. Analysing the mechanism of alpha-synuclein fibril formation, helped us in elucidating the passage of an intermediate at 75 muM concentration of escitalopram. Fibrils of alpha-synuclein were obtained at 100 muM concentration of escitalopram. Inhibition of alpha-synuclein fibrillation was brought about by a polyphenolic acid known as Caffeic acid which acted in a concentration dependent manner ranging from 10 to 60 muM. Maximum inhibition was achieved at a concentration of 60 muM. Fibrillation of alpha-synuclein in presence of escitalopram gives us clue for the negative effects of antidepressant. Inhibitory activity of Caffeic acid against alpha-synuclein fibrillation may guide us in designing novel therapeutic drugs for PD.
Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice.[Pubmed:16644902]
J Pharmacol Exp Ther. 2006 Aug;318(2):476-83.
This study investigated the blood glucose-lowering effect and antioxidant capacity of Caffeic acid in C57BL/KsJ-db/db mice. Caffeic acid induced a significant reduction of the blood glucose and glycosylated hemoglobin levels than the control group. The plasma insulin, C-peptide, and leptin levels in Caffeic acid group were significantly higher than those of the control group, whereas the plasma glucagon level was lower. Increased plasma insulin by Caffeic acid was attributable to an antidegenerative effect on the islets. Caffeic acid also markedly increased glucokinase activity and its mRNA expression and glycogen content and simultaneously lowered glucose-6-phosphatase and phosphoenolpyruvate carboxykinase activities and their respective mRNA expressions, accompanied by a reduction in the glucose transporter 2 expression in the liver. In contrast to the hepatic glucose transporter 2, adipocyte glucose transporter 4 expression was greater than the control group. In addition, Caffeic acid significantly increased superoxide dismutase, catalase, and glutathione peroxidase activities and their respective mRNA levels, while lowering the hydrogen peroxide and thiobarbituric acid reactive substances levels in the erythrocyte and liver of db/db mice. These results indicate that Caffeic acid exhibits a significant potential as an antidiabetic agent by suppressing a progression of type 2 diabetic states that is suggested by an attenuation of hepatic glucose output and enhancement of adipocyte glucose uptake, insulin secretion, and antioxidant capacity.


