BAM7BAX activator,direct and selective CAS# 331244-89-4 |
- Bax inhibitor peptide, negative control
Catalog No.:BCC2395
CAS No.:1315378-74-5
- Bax channel blocker
Catalog No.:BCC2392
CAS No.:335165-68-9
- PRIMA-1
Catalog No.:BCC2413
CAS No.:5608-24-2
- Bax inhibitor peptide V5
Catalog No.:BCC2394
CAS No.:579492-81-2
- Bax inhibitor peptide P5
Catalog No.:BCC2393
CAS No.:579492-83-4
Quality Control & MSDS
Number of papers citing our products

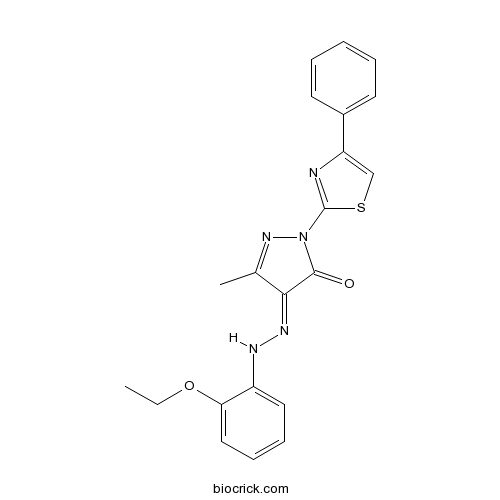
Chemical structure
3D structure
| Cas No. | 331244-89-4 | SDF | Download SDF |
| PubChem ID | 5754137 | Appearance | Powder |
| Formula | C21H19N5O2S | M.Wt | 405.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 5 mg/mL (12.33 mM; Need ultrasonic) | ||
| Chemical Name | (4E)-4-[(2-ethoxyphenyl)hydrazinylidene]-5-methyl-2-(4-phenyl-1,3-thiazol-2-yl)pyrazol-3-one | ||
| SMILES | CCOC1=CC=CC=C1NN=C2C(=NN(C2=O)C3=NC(=CS3)C4=CC=CC=C4)C | ||
| Standard InChIKey | KOCVKGYKBLJEPK-LYBHJNIJSA-N | ||
| Standard InChI | InChI=1S/C21H19N5O2S/c1-3-28-18-12-8-7-11-16(18)23-24-19-14(2)25-26(20(19)27)21-22-17(13-29-21)15-9-5-4-6-10-15/h4-13,23H,3H2,1-2H3/b24-19+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bax activator (EC50 = 3.3 μM); binds at the BH3-binding site. Selective for Bax over other antiapoptotic and proapoptotic proteins. Triggers Bax oligomerization in vitro; induces Bax-mediated apoptosis in mouse embryonic fibroblasts. |

BAM7 Dilution Calculator

BAM7 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4663 mL | 12.3314 mL | 24.6627 mL | 49.3255 mL | 61.6568 mL |
| 5 mM | 0.4933 mL | 2.4663 mL | 4.9325 mL | 9.8651 mL | 12.3314 mL |
| 10 mM | 0.2466 mL | 1.2331 mL | 2.4663 mL | 4.9325 mL | 6.1657 mL |
| 50 mM | 0.0493 mL | 0.2466 mL | 0.4933 mL | 0.9865 mL | 1.2331 mL |
| 100 mM | 0.0247 mL | 0.1233 mL | 0.2466 mL | 0.4933 mL | 0.6166 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BAM7 is a direct and selective activator of BAX with IC50 value of 3.3uM [1].
In the competitive fluorescence polarization assay (FPA), BAM7 competes with FITC–BIM SAHB for BAX binding site(BH3) in a dose dependent manner. BAM7 shows no antiapoptotic or BAKΔC competitive binding interactions even at 50 μM dosing, revealing a remarkable selectivity of BAM7 for BAX. The interaction between BAM7 and BAX at the very surface induces the characteristic structural changes that yield functional BAX oligomerization. In the in vitro assay, BAM7 induces BAX-dependent cell death but not the cells with BAK. BAM7 could be developed to a new generation of apoptotic modulators that directly activate BCL-2 family executioner proteins in cancer
and other diseases driven by pathologic apoptotic blockades [1].
References:
[1] Evripidis Gavathiotis, Denis E Reyna, Joseph A Bellairs, Elizaveta S Leshchiner, and Loren D Walensky. Direct and selective small-molecule activation of proapoptotic BAX. Nat Chem Biol. 2012 July ; 8(7): 639–645.
- Stephavanine
Catalog No.:BCN5253
CAS No.:33116-33-5
- PT 1
Catalog No.:BCC7846
CAS No.:331002-70-1
- IQ 1
Catalog No.:BCC7965
CAS No.:331001-62-8
- Caffeic acid
Catalog No.:BCN5979
CAS No.:331-39-5
- AS 1269574
Catalog No.:BCC7878
CAS No.:330981-72-1
- Betrixaban
Catalog No.:BCC5118
CAS No.:330942-05-7
- Amitraz
Catalog No.:BCC8816
CAS No.:33089-61-1
- MRT 10
Catalog No.:BCC7950
CAS No.:330829-30-6
- PCI 29732
Catalog No.:BCC4100
CAS No.:330786-25-9
- Avanafil
Catalog No.:BCC2288
CAS No.:330784-47-9
- Paclitaxel
Catalog No.:BCN4650
CAS No.:33069-62-4
- KH 7
Catalog No.:BCC7787
CAS No.:330676-02-3
- LG 101506
Catalog No.:BCC7696
CAS No.:331248-11-4
- Boc-D-Phg-OH
Catalog No.:BCC3315
CAS No.:33125-05-2
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- trans-2,3,4-Trimethoxycinnamic acid
Catalog No.:BCN5035
CAS No.:33130-03-9
- CFM 4
Catalog No.:BCC8017
CAS No.:331458-02-7
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- DMPO
Catalog No.:BCC7684
CAS No.:3317-61-1
- Bisdemethoxycurcumin
Catalog No.:BCN5975
CAS No.:33171-05-0
- 10-Demethoxy-10-(diethylamino)colchicine
Catalog No.:BCC8164
CAS No.:6962-03-4
- ZM 447439
Catalog No.:BCC2169
CAS No.:331771-20-1
- 2-(N,N-Dimethylamino)acetophenone
Catalog No.:BCN1747
CAS No.:3319-03-7
- 2',4'-Dihydroxy-7-methoxy-8-prenylflavan
Catalog No.:BCN6844
CAS No.:331954-16-6
beta-amylase-like proteins function as transcription factors in Arabidopsis, controlling shoot growth and development.[Pubmed:21487098]
Plant Cell. 2011 Apr;23(4):1391-403.
Plants contain beta-amylase-like proteins (BAMs; enzymes usually associated with starch breakdown) present in the nucleus rather than targeted to the chloroplast. They possess BRASSINAZOLE RESISTANT1 (BZR1)-type DNA binding domains--also found in transcription factors mediating brassinosteroid (BR) responses. The two Arabidopsis thaliana BZR1-BAM proteins (BAM7 and BAM8) bind a cis-regulatory element that both contains a G box and resembles a BR-responsive element. In protoplast transactivation assays, these BZR1-BAMs activate gene expression. Structural modeling suggests that the BAM domain's glucan binding cleft is intact, but the recombinant proteins are at least 1000 times less active than chloroplastic beta-amylases. Deregulation of BZR1-BAMs (the BAM7bam8 double mutant and BAM8-overexpressing plants) causes altered leaf growth and development. Of the genes upregulated in plants overexpressing BAM8 and downregulated in BAM7bam8 plants, many carry the cis-regulatory element in their promoters. Many genes that respond to BRs are inversely regulated by BZR1-BAMs. We propose a role for BZR1-BAMs in controlling plant growth and development through crosstalk with BR signaling. Furthermore, we speculate that BZR1-BAMs may transmit metabolic signals by binding a ligand in their BAM domain, although diurnal changes in the concentration of maltose, a candidate ligand produced by chloroplastic beta-amylases, do not influence their transcription factor function.
Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson's disease: involvement of antioxidative enzymes induction.[Pubmed:25446857]
Chem Biol Interact. 2015 Jan 5;225:40-6.
The neuroprotective effects of carnosic acid (CA), a phenolic diterpene isolated from rosemary (Rosmarinus officinalis), have been widely investigated in recent years, however, its protection in in vivo still unclear. In this study, we investigated the behavioral activity and neuroprotective effects of CA in a rat model of Parkinson's disease (PD) induced by 6-hydroxydopamine (6-OHDA). Rats were treated with 20mg/kg body weight of CA for 3 weeks before 6-OHDA exposure. Results indicated that CA improved the locomotor activity and reduced the apomorphine-caused rotation in 6-OHDA-stimulated rats. Significant protection against lipid peroxidation and GSH reduction was observed in the 6-OHDA rats pretreated with CA. Pretreatment with CA increased the protein expression of gamma-glutamate-cysteine ligase catalytic subunit, gamma-glutamate-cysteine ligase modifier subunit, superoxide dismutase, and glutathione reductase compared with 6-OHDA-stimulated rats and SH-SY5Y cells. Immunoblots showed that the reduction of the Bcl-2/Bax ratio, the induction of caspase 3 cleavage, and the induction of poly(ADP-ribose) polymerase (PARP) cleavage by 6-OHDA was reversed in the presence of SB203580 (a p38 inhibitor) or SP600125 (a JNK inhibitor) in SH-SY5Y cells. Rats treated with CA reversed the 6-OHDA-mediated the activation of c-Jun NH2-terminal kinase and p38, the down-regulation of the Bcl-2/Bax ratio, the up-regulation of cleaved caspase 3/caspase 3 and cleaved PARP/PARP ratio, and the down-regulation of tyrosine hydroxylase protein. However, BAM7, an activator of Bax, attenuated the effect of CA on apoptosis in SH-SY5Y cells. These results suggest that CA protected against 6-OHDA-induced neurotoxicity is attributable to its anti-apoptotic and anti-oxidative action. The present findings may help to clarify the possible mechanisms of rosemary in the neuroprotection of PD.
The Enzyme-Like Domain of Arabidopsis Nuclear beta-Amylases Is Critical for DNA Sequence Recognition and Transcriptional Activation.[Pubmed:24748042]
Plant Cell. 2014 Apr;26(4):1746-1763.
Plant BZR1-BAM transcription factors contain a beta-amylase (BAM)-like domain, characteristic of proteins involved in starch breakdown. The enzyme-derived domains appear to be noncatalytic, but they determine the function of the two Arabidopsis thaliana BZR1-BAM isoforms (BAM7 and BAM8) during transcriptional initiation. Removal or swapping of the BAM domains demonstrates that the BAM7 BAM domain restricts DNA binding and transcriptional activation, while the BAM8 BAM domain allows both activities. Furthermore, we demonstrate that BAM7 and BAM8 interact on the protein level and cooperate during transcriptional regulation. Site-directed mutagenesis of residues in the BAM domain of BAM8 shows that its function as a transcriptional activator is independent of catalysis but requires an intact substrate binding site, suggesting it may bind a ligand. Microarray experiments with plants overexpressing truncated versions lacking the BAM domain indicate that the pseudo-enzymatic domain increases selectivity for the preferred cis-regulatory element BBRE (BZR1-BAM Responsive Element). Side specificity toward the G-box may allow crosstalk to other signaling networks. This work highlights the importance of the enzyme-derived domain of BZR1-BAMs, supporting their potential role as metabolic sensors.
Bax Activation Blocks Self-Renewal and Induces Apoptosis of Human Glioblastoma Stem Cells.[Pubmed:28368610]
ACS Chem Neurosci. 2018 Jan 17;9(1):85-99.
Glioblastoma (GBM) is characterized by a poor response to conventional chemotherapeutic agents, attributed to the insurgence of drug resistance mechanisms and to the presence of a subpopulation of glioma stem cells (GSCs). GBM cells and GSCs present, among others, an overexpression of antiapoptotic proteins and an inhibition of pro-apoptotic ones, which help to escape apoptosis. Among pro-apoptotic inducers, the Bcl-2 family protein Bax has recently emerged as a promising new target in cancer therapy along with first BAX activators (BAM7, Compound 106, and SMBA1). Herein, a derivative of BAM-7, named BTC-8, was employed to explore the effects of Bax activation in different human GBM cells and in their stem cell subpopulation. BTC-8 inhibited GBM cell proliferation, arrested the cell cycle, and induced apoptosis through the induction of mitochondrial membrane permeabilization. Most importantly, BTC-8 blocked proliferation and self-renewal of GSCs and induced their apoptosis. Notably, BTC-8 was demonstrated to sensitize both GBM cells and GSCs to the alkylating agent Temozolomide. Overall, our findings shed light on the effects and the relative molecular mechanisms related to Bax activation in GBM, and they suggest Bax-targeting compounds as promising therapeutic tools against the GSC reservoir.
Direct and selective small-molecule activation of proapoptotic BAX.[Pubmed:22634637]
Nat Chem Biol. 2012 Jul;8(7):639-45.
BCL-2 family proteins are key regulators of the apoptotic pathway. Antiapoptotic members sequester the BCL-2 homology 3 (BH3) death domains of proapoptotic members such as BAX to maintain cell survival. The antiapoptotic BH3-binding groove has been successfully targeted to reactivate apoptosis in cancer. We recently identified a geographically distinct BH3-binding groove that mediates direct BAX activation, suggesting a new strategy for inducing apoptosis by flipping BAX's 'on switch'. Here we applied computational screening to identify a BAX activator molecule that directly and selectively activates BAX. We demonstrate by NMR and biochemical analyses that the molecule engages the BAX trigger site and promotes the functional oligomerization of BAX. The molecule does not interact with the BH3-binding pocket of antiapoptotic proteins or proapoptotic BAK and induces cell death in a BAX-dependent fashion. To our knowledge, we report the first gain-of-function molecular modulator of a BCL-2 family protein and demonstrate a new paradigm for pharmacologic induction of apoptosis.


