GF 109203XProtein kinase C,MLCK,PKG and PKA inhibitor CAS# 133052-90-1 |
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
Number of papers citing our products

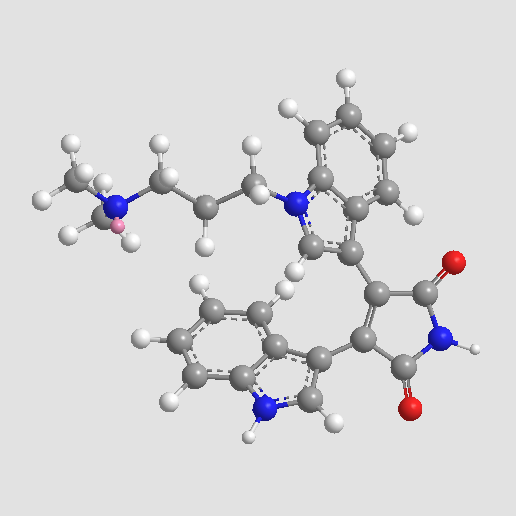
Chemical structure
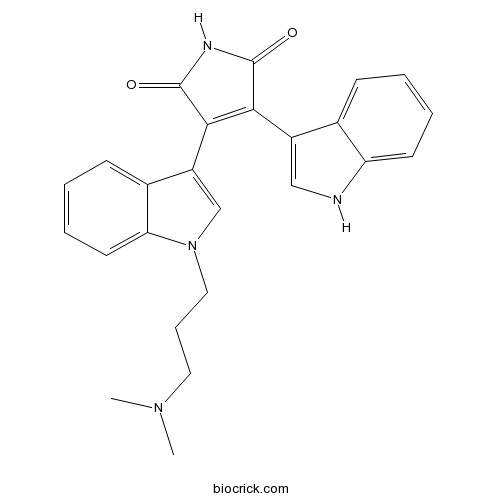
3D structure
| Cas No. | 133052-90-1 | SDF | Download SDF |
| PubChem ID | 2396 | Appearance | Powder |
| Formula | C25H24N4O2 | M.Wt | 412.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Gö 6850, Bisindolylmaleimide I | ||
| Solubility | DMSO : ≥ 32 mg/mL (77.58 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 3-[1-[3-(dimethylamino)propyl]indol-3-yl]-4-(1H-indol-3-yl)pyrrole-2,5-dione | ||
| SMILES | CN(C)CCCN1C=C(C2=CC=CC=C21)C3=C(C(=O)NC3=O)C4=CNC5=CC=CC=C54 | ||
| Standard InChIKey | QMGUOJYZJKLOLH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H24N4O2/c1-28(2)12-7-13-29-15-19(17-9-4-6-11-21(17)29)23-22(24(30)27-25(23)31)18-14-26-20-10-5-3-8-16(18)20/h3-6,8-11,14-15,26H,7,12-13H2,1-2H3,(H,27,30,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Very potent and selective inhibitor of protein kinase C, selective for the α and β1 isoforms (IC50 values are 0.0084, 0.0180, 0.210, 0.132, and 5.8 μM for α, β1, δ, ε and ζ isoforms respectively). Selective over MLCK, PKG and PKA (IC50 values are 0.6, 4.6, and 33 μM respectively). Potent antagonist at the 5-HT3 receptor (Ki = 29.5 nM). Anti-inflammatory in vivo. |

GF 109203X Dilution Calculator

GF 109203X Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4243 mL | 12.1215 mL | 24.243 mL | 48.486 mL | 60.6075 mL |
| 5 mM | 0.4849 mL | 2.4243 mL | 4.8486 mL | 9.6972 mL | 12.1215 mL |
| 10 mM | 0.2424 mL | 1.2122 mL | 2.4243 mL | 4.8486 mL | 6.0608 mL |
| 50 mM | 0.0485 mL | 0.2424 mL | 0.4849 mL | 0.9697 mL | 1.2122 mL |
| 100 mM | 0.0242 mL | 0.1212 mL | 0.2424 mL | 0.4849 mL | 0.6061 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GF 109203X is a potent and selective inhibitor of protein kinase C [1].
Protein kinase C (PKC) is a family of protein kinase enzymes that are involved in controlling the function of other proteins through the phosphorylation of serine and threonine amino acid residues on target proteins. PKC enzymes are activated by increases in the concentration of Ca2+ or diacylglycerol (DAG).
GF 109203X is a competitive inhibitor with Ki value of 14 nM. It inhibited PKC with IC50 values of 0.020, 0.017, 0.016, 0.020 μM for α, βI, βII and γ, respectively. In human platelets and Swiss 3T3 fibroblasts, GF 109203X significantly inhibited PKC-mediated phosphorylations with Mr of 47000 and 80000 in platelets and Swiss 3T3 cells, respectively. Also, GF 109203X inhibited collagen-triggered ATP secretion as well as α- thrombin- and collagen- induced platelet aggregation [1]. GF 109203X selectively inhibited PKC activity extracted from either fibroblasts or keratinocytes with IC50 values of 0.01 μM and 0.4 μM, respectively. Also, GF 109203X inhibited the expression of c-fos and c-jun, which involved in the cellular differentiation process [2].
References:
[1]. Toullec D, Pianetti P, Coste H, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem, 1991, 266(24): 15771-15781.
[2]. Le Panse R, Coulomb B, Mitev V, et al. Differential modulation of human fibroblast and keratinocyte growth by the protein kinase C inhibitor GF 109203X. Mol Pharmacol, 1994, 46(3): 445-451.
- LY 225910
Catalog No.:BCC6891
CAS No.:133040-77-4
- Ethacrynic acid - d5
Catalog No.:BCC7987
CAS No.:1330052-59-9
- (+)-SK&F 10047 hydrochloride
Catalog No.:BCC6928
CAS No.:133005-41-1
- 13-Epijhanol
Catalog No.:BCN4713
CAS No.:133005-15-9
- Trichlormethiazide
Catalog No.:BCC4872
CAS No.:133-67-5
- 3-Indolebutyric acid (IBA)
Catalog No.:BCC6491
CAS No.:133-32-4
- Asarinin
Catalog No.:BCN2769
CAS No.:133-05-1
- (-)-Asarinin
Catalog No.:BCN2290
CAS No.:133-04-0
- Fmoc-Lys(Fmoc)-OPfp
Catalog No.:BCC3522
CAS No.:132990-14-8
- Mequindox
Catalog No.:BCC9021
CAS No.:13297-17-1
- Macrocarpal A
Catalog No.:BCN6178
CAS No.:132951-90-7
- Rifampin
Catalog No.:BCC4839
CAS No.:13292-46-1
- Go 6983
Catalog No.:BCC3705
CAS No.:133053-19-7
- ZD 7288
Catalog No.:BCC6884
CAS No.:133059-99-1
- BIM 23052
Catalog No.:BCC5945
CAS No.:133073-82-2
- Fmoc-D-Lys(Boc)-OPfp
Catalog No.:BCC3527
CAS No.:133083-36-0
- Crassifoline methine
Catalog No.:BCN1793
CAS No.:133084-00-1
- 3PO
Catalog No.:BCC5616
CAS No.:13309-08-5
- Darifenacin
Catalog No.:BCC1516
CAS No.:133099-04-4
- Darifenacin HBr
Catalog No.:BCC4567
CAS No.:133099-07-7
- Flutamide
Catalog No.:BCC4364
CAS No.:13311-84-7
- Epimedin B1
Catalog No.:BCN8199
CAS No.:133137-58-3
- GR 73632
Catalog No.:BCC5801
CAS No.:133156-06-6
- Oxypeucedan hydrate
Catalog No.:BCN8372
CAS No.:133164-11-1
The protein kinase C inhibitors bisindolylmaleimide I (GF 109203x) and IX (Ro 31-8220) are potent inhibitors of glycogen synthase kinase-3 activity.[Pubmed:10556511]
FEBS Lett. 1999 Nov 5;460(3):433-6.
Here we report that the widely used protein kinase C inhibitors, bisindolylmaleimide I and IX, are potent inhibitors of glycogen synthase kinase-3 (GSK-3). Bisindolylmaleimide I and IX inhibited GSK-3 in vitro, when assayed either in cell lysates (IC(50) 360 nM and 6.8 nM, respectively) or in GSK-3beta immunoprecipitates (IC(50) 170 nM and 2.8 nM, respectively) derived from rat epididymal adipocytes. Pretreatment of adipocytes with bisindolylmaleimide I (5 microM) and IX (2 microM) reduced GSK-3 activity in total cell lysates, to 25.1+/-4.3% and 12.9+/-3.0% of control, respectively. By contrast, bisindolylmaleimide V (5 microM), which lacks the functional groups present on bisindolylmaleimide I and IX, had little apparent effect. We propose that bisindolylmaleimide I and IX can directly inhibit GSK-3, and that this may explain some of the previously reported insulin-like effects on glycogen synthase activity.
Effects of the PKC inhibitors chelerythrine and bisindolylmaleimide I (GF 109203X) on delayed rectifier K+ currents.[Pubmed:21120453]
Naunyn Schmiedebergs Arch Pharmacol. 2011 Feb;383(2):141-8.
Protein kinase C (PKC) inhibitors are useful tools for studying PKC-dependent regulation of ion channels. For this purpose, high PKC specificity is a basic requirement excluding any direct interaction between the PKC inhibitor and the ion channel. In the present study, the effects of two frequently applied PKC inhibitors, chelerythine and bisindolylmaleimide I, were studied on the rapid and slow components of the delayed rectifier K(+) current (I(Kr) and I(Ks)) in canine ventricular cardiomyocytes and on the human ether-a-go-go-related gene (hERG) channels expressed in human embryonic kidney (HEK) cells. The whole cell version of the patch clamp technique was used in all experiments. Chelerythrine and bisindolylmaleimide I (both 1 muM) suppressed I(Kr) in canine ventricular cells. This inhibition developed rapidly, suggesting a direct drug-channel interaction. In HEK cells heterologously expressing hERG channels, chelerythrine and bisindolylmaleimide I blocked hERG current in a concentration-dependent manner, having EC(50) values of 0.11 +/- 0.01 and 0.76 +/- 0.04 muM, respectively. Both chelerythrine and bisindolylmaleimide I strongly modified gating kinetics of hERG--voltage dependence of activation was shifted towards more negative voltages and activation was accelerated. Deactivation was slowed by bisindolylmaleimide I but not by chelerythrine. I(Ks) was not significantly altered by bisindolylmaleimide I and chelerythrine. No significant effect of 0.1 muM bisindolylmaleimide I or 0.1 muM PMA (PKC activator) was observed on I(Kr) arguing against significant contribution of PKC to regulation of I(Kr). It is concluded that neither chelerythrine nor bisindolylmaleimide I is suitable for selective PKC blockade due to their direct blocking actions on the hERG channel.
The protein kinase C inhibitors Ro 318220 and GF 109203X are equally potent inhibitors of MAPKAP kinase-1beta (Rsk-2) and p70 S6 kinase.[Pubmed:9037179]
FEBS Lett. 1997 Feb 3;402(2-3):121-3.
The protein kinase C (PKC) inhibitors Ro 318220 and GF 109203X have been used in over 350 published studies to investigate the physiological roles of PKC. Here we demonstrate that these inhibitors are not selective for PKC isoforms as was previously assumed. Ro 318220 inhibited MAPKAP kinase-1beta (also known as Rsk-2) in vitro (IC50 3nM) more potently than it inhibited mixed PKC isoforms (IC50 5 nM), and it also inhibited p70 S6 kinase (IC50 15 nM). GF 109203X also potently inhibited MAPKAP kinase-1beta (IC50 50 nM) and p70 S6 kinase (IC50 100 nM) with similar potency to PKC isoforms (IC50 30 nM). The inhibition of MAPKAP kinase-1beta, p70 S6 kinase, and probably other protein kinases, may explain many of the effects previously attributed to PKC.
GF 109203X, a selective inhibitor of protein kinase C, impairs retention performance in an operant task.[Pubmed:10511444]
Neuroreport. 1999 Sep 9;10(13):2805-9.
The effects of post-training administration of GF 109203X (5 and 50 ng i.c.v.), a selective inhibitor of protein kinase C, on retention performance were investigated in a positively reinforced lever press task, in male Swiss mice. Both doses of GF 109203X suppressed the spontaneous improvement of performance observed in control animals between the last 5 min of the acquisition session and the first 5 min of the retention session 24 h later. GF 109203X had no effect on food intake and locomotor activity. These data suggest that GF 109203X selectively interferes with mechanisms underlying post-training organization of information and that protein kinase C is involved in this memory process.
Heat shock protein 90-mediated inactivation of nuclear factor-kappaB switches autophagy to apoptosis through becn1 transcriptional inhibition in selenite-induced NB4 cells.[Pubmed:21346199]
Mol Biol Cell. 2011 Apr 15;22(8):1167-80.
Autophagy can protect cells while also contributing to cell damage, but the precise interplay between apoptosis and autophagy and the contribution of autophagy to cell death are still not clear. Previous studies have shown that supranutritional doses of sodium selenite promote apoptosis in human leukemia NB4 cells. Here, we report that selenite treatment triggers opposite patterns of autophagy in the NB4, HL60, and Jurkat leukemia cell lines during apoptosis and provide evidence that the suppressive effect of selenite on autophagy in NB4 cells is due to the decreased expression of the chaperone protein Hsp90 (heat shock protein 90), suggesting a novel regulatory function of Hsp90 in apoptosis and autophagy. Excessive or insufficient expression indicates that Hsp90 protects NB4 cells from selenite-induced apoptosis, and selenite-induced decreases in the expression of Hsp90, especially in NB4 cells, inhibit the activities of the IkappaB kinase/nuclear factor-kappaB (IKK/NF-kappaB) signaling pathway, leading to less nuclear translocation and inactivation of NF-kappaB and the subsequent weak binding of the becn1 promoter, which facilitates the transition from autophagy to apoptosis. Taken together, our observations provide novel insights into the mechanisms underlying the balance between apoptosis and autophagy, and we also identified Hsp90-NF-kappaB-Beclin1 as a potential biological pathway for signaling the switch from autophagy to apoptosis in selenite-treated NB4 cells.
Competitive antagonism of the mouse 5-hydroxytryptamine3 receptor by bisindolylmaleimide I, a "selective" protein kinase C inhibitor.[Pubmed:10381762]
J Pharmacol Exp Ther. 1999 Jul;290(1):76-82.
We examined the effects of several protein kinase C (PKC) inhibitors on the murine 5-hydroxytryptamine3 (5-HT3) receptor to determine whether they acted directly on the receptor. The 5-HT-evoked currents in Xenopus laevis oocytes expressing the recombinant 5-HT3 receptor were measured with the two-electrode voltage-clamp technique. The PKC inhibitors bisindolylmaleimide I (BIM, GF109203x) and staurosporine, but not calphostin C or chelerythrine, decreased the 5-HT3 receptor-mediated currents when coapplied with 5-HT. BIM blocked 0.5 microM 5-HT-elicited currents with an IC50 value of 7 nM, whereas in the presence of 5 microM staurosporine, 42% inhibition of 0.5 microM 5-HT-mediated currents was observed. Increasing concentrations of BIM resulted in a rightward shift of the 5-HT concentration-response curve, without altering efficacy. A Schild plot was generated, which had a slope of -1.01, suggesting competitive antagonism. The Ki value of BIM was determined to be 29 nM. To confirm competitive antagonism, a competitive binding assay was performed on Sf21 insect cells infected with the mouse 5-HT3 receptor cDNA in a baculovirus expression vector. BIM completely displaced binding of the selective 5-HT3 receptor antagonist [3H]GR65630. BIM bound to the 5-HT3 receptor with a Ki value of 61 nM, which was slightly less potent than that of the selective 5-HT3 receptor antagonist MDL72222 (27 nM). The PKC inhibitor BIM is a potent competitive antagonist at the 5-HT3 receptor.
Anti-inflammatory properties of Go 6850: a selective inhibitor of protein kinase C.[Pubmed:7473193]
J Pharmacol Exp Ther. 1995 Nov;275(2):995-1002.
Protein kinase C (PKC) regulates a variety of signal transduction events implicated in the pathogenesis of inflammation, including the biosynthesis of inflammatory cytokines and superoxide and the activation of phospholipase A2. Because of the significant role of PKC in these inflammatory processes, we evaluated a specific and potent inhibitor of C kinase for efficacy in several in vitro and in vivo murine models of inflammation. Unlike the relatively nonspecific kinase inhibitor staurosporine, the bisindolylmaleimide 3-[1-[-3-(dimethylaminopropyl]-1H-indol-3-yl]- 4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione monohydrochloride (Go 6850) demonstrated increased selectivity for C kinase in purified enzyme assays (respective IC50 values (microM) for Go 6850 and staurosporine: protein kinase C (0.032, 0.009); myosin light-chain kinase (0.6, 0.01); protein kinase G (4.6, 0.018); protein kinase A (33, 0.04); tyrosine kinase1 (94, 0.4); tyrosine kinase2 (> 100, > 1)). Topically applied Go 6850 inhibited phorbol myristate acetate-induced edema, neutrophil influx and vascular permeability in murine epidermis in a dose- and time-dependent manner at levels comparable to indomethacin. In a murine model of delayed type hypersensitivity, Go 6850 inhibited dinitrofluorobenzene-induced contact dermatitis with and ID50 value of 150 micrograms/ear. Cellular studies in mouse peritoneal macrophages demonstrated that Go 6850 was a potent inhibitor of phorbol myristate acetate-induced prostaglandin E2 production. Superoxide production in phorbol myristate acetate-stimulated murine neutrophils was also inhibited by Go 6850 (IC50 = 88 nM).(ABSTRACT TRUNCATED AT 250 WORDS)
Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976.[Pubmed:8486620]
J Biol Chem. 1993 May 5;268(13):9194-7.
Indolocarbazoles have been identified as novel inhibitors of protein kinase C (PKC), with Go 6976 as one of its most potent and selective representatives. Recombinant PKC isozymes alpha, beta 1, delta, epsilon, and zeta were used in in vitro kinase assays to investigate Go 6976 with respect to isozyme-specific PKC inhibition. Go 6850, identical with GF 109203X, another PKC-specific kinase inhibitor, was included in this study as a reference compound. Nanomolar concentrations of the indolocarbazole Go 6976 inhibited the Ca(2+)-dependent isozymes alpha and beta 1, whereas even micromolar concentration of Go 6976 had no effect on the kinase activity of the Ca(2+)-independent PKC subtypes delta, epsilon, and zeta. In contrast, the bisindolymaleimide Go 6850 inhibited all PKC isozymes, however, with a ranked order of potency (alpha > beta 1 > epsilon > delta > zeta). Kinetic analysis revealed that PKC inhibition by Go 6976 was competitive with respect to ATP, non-competitive with respect to the protein substrate, and mixed type with respect to phosphatidylserine. Further experiments in the presence of different amounts of free Ca2+ indicated that interference with Ca2+ or its binding site is not responsible for the differential inhibition of PKC isozymes by Go 6976.
The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C.[Pubmed:1874734]
J Biol Chem. 1991 Aug 25;266(24):15771-81.
Staurosporine is the most potent inhibitor of protein kinase C (PKC) described in the literature with a half-maximal inhibitory concentration (IC50) of 10 nM. Nevertheless, this natural product is poorly selective when assayed against other protein kinases. In order to obtain specific PKC inhibitors, a series of bisindolylmaleimides has been synthesized. Structure-activity relationship studies allowed the determination of the substructure responsible for conferring high potency and lack of selectivity in the staurosporine molecule. Several aminoalkyl bisindolylmaleimides were found to be potent and selective PKC inhibitors (IC50 values from 5 to 70 nM). Among these compounds GF 109203X has been chosen for further studies aiming at the characterization of this chemical family. GF 109203X was a competitive inhibitor with respect to ATP (Ki = 14 +/- 3 NM) and displayed high selectivity for PKC as compared to five different protein kinases. We further determined the potency and specificity of GF 109203X in two cellular models: human platelets and Swiss 3T3 fibroblasts. GF 109203X efficiently prevented PKC-mediated phosphorylations of an Mr = 47,000 protein in platelets and of an Mr = 80,000 protein in Swiss 3T3 cells. In contrast, in the same models, the PKC inhibitor failed to prevent PKC-independent phosphorylations. GF 109203X inhibited collagen- and alpha-thrombin-induced platelet aggregation as well as collagen-triggered ATP secretion. However, ADP-dependent reversible aggregation was not modified. In Swiss 3T3 fibroblasts, GF 109203X reversed the inhibition of epidermal growth factor binding induced by phorbol 12,13-dibutyrate and prevented [3H] thymidine incorporation into DNA, only when this was elicited by growth promoting agents which activate PKC. Our results illustrate the potential of GF 109203X as a tool for studying the involvement of PKC in signal transduction pathways.


