Gatifloxacin hydrochlorideCAS# 121577-32-0 |
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
- Ellagic acid
Catalog No.:BCN5533
CAS No.:476-66-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 121577-32-0 | SDF | Download SDF |
| PubChem ID | 17956339 | Appearance | Powder |
| Formula | C19H23ClFN3O4 | M.Wt | 411.86 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AM 1155 hydrochloride; BMS 206584-01 hydrochloride; PD 135432 hydrochloride | ||
| Solubility | Soluble in DMSO | ||
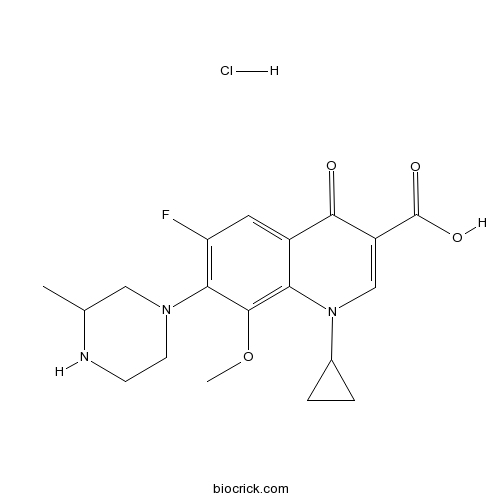
| Chemical Name | 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid;hydrochloride | ||
| SMILES | CC1CN(CCN1)C2=C(C=C3C(=C2OC)N(C=C(C3=O)C(=O)O)C4CC4)F.Cl | ||
| Standard InChIKey | GQYBNVXJQVIRGC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H22FN3O4.ClH/c1-10-8-22(6-5-21-10)16-14(20)7-12-15(18(16)27-2)23(11-3-4-11)9-13(17(12)24)19(25)26;/h7,9-11,21H,3-6,8H2,1-2H3,(H,25,26);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Gatifloxacin (hydrochloride) is an antibiotic of the fourth-generation fluoroquinolone family, it inhibits the bacterial enzymes DNA gyrase and topoisomerase IV.
Target: Antibacterial
Gatifloxacin (hydrochloride) is the hydrochloride salt of Gatifloxacin which is an antibiotic of the fourth-generation fluoroquinolone family, that like other members of that family, inhibits the bacterial enzymes DNA gyrase and topoisomerase IV. Gatifloxacin had activity equal to that of tosufloxacin and activity more potent than those of norfloxacin, ofloxacin, ciprofloxacin, and sparfloxacin against the second-step mutants (grlA gyrA; gatifloxacin MIC range, 1.56 to 3.13 microg/ml) and had the most potent activity against the third-step mutants (grlA gyrA grlA; gatifloxacin MIC range, 1.56 to 6.25 microg/ml), suggesting that gatifloxacin possesses the most potent inhibitory activity against singly mutated topo IV and singly mutated DNA gyrase among the quinolones tested [1].
Ophthalmic gatifloxacin 0.3% is at least as effective as ciprofloxacin at healing corneal ulcers infected with Pseudomonas aeruginosa when gatifloxacin is administered less frequently than ciprofloxacin. Trends favored gatifloxacin in fluorescein retention scores [2].
Clinical indications: Bacterial infection
FDA Approved Date:
Toxicity: Hepatotoxicity; Acute pancreatitis [3]; Torsades de pointes [4] References: | |||||

Gatifloxacin hydrochloride Dilution Calculator

Gatifloxacin hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.428 mL | 12.14 mL | 24.2801 mL | 48.5602 mL | 60.7002 mL |
| 5 mM | 0.4856 mL | 2.428 mL | 4.856 mL | 9.712 mL | 12.14 mL |
| 10 mM | 0.2428 mL | 1.214 mL | 2.428 mL | 4.856 mL | 6.07 mL |
| 50 mM | 0.0486 mL | 0.2428 mL | 0.4856 mL | 0.9712 mL | 1.214 mL |
| 100 mM | 0.0243 mL | 0.1214 mL | 0.2428 mL | 0.4856 mL | 0.607 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Gatifloxacin (hydrochloride) is an antibiotic of the fourth-generation fluoroquinolone family, it inhibits the bacterial enzymes DNA gyrase and topoisomerase IV.
- NBI 27914 hydrochloride
Catalog No.:BCC7124
CAS No.:1215766-76-9
- CP-809101 hydrochloride
Catalog No.:BCC1499
CAS No.:1215721-40-6
- RS 100329 hydrochloride
Catalog No.:BCC5741
CAS No.:1215654-26-4
- SB 242084
Catalog No.:BCC5949
CAS No.:1215566-78-1
- CFM 1571 hydrochloride
Catalog No.:BCC5924
CAS No.:1215548-30-3
- RG2833
Catalog No.:BCC1893
CAS No.:1215493-56-3
- GR 144053 trihydrochloride
Catalog No.:BCC6998
CAS No.:1215333-48-4
- SR 58611A hydrochloride
Catalog No.:BCC7833
CAS No.:121524-09-2
- Salvianolic acid B; Lithospermic acid B; Danfensuan B
Catalog No.:BCC8249
CAS No.:121521-90-2
- PF-04991532
Catalog No.:BCC8094
CAS No.:1215197-37-7
- 5-Benzyloxyindole
Catalog No.:BCC8742
CAS No.:1215-59-4
- Daclatasvir (BMS-790052)
Catalog No.:BCC2533
CAS No.:1214735-16-6
- DMCM hydrochloride
Catalog No.:BCC7560
CAS No.:1215833-62-7
- Valspodar
Catalog No.:BCC2027
CAS No.:121584-18-7
- 6-Demethoxy-9'-deoxycleomiscosin A
Catalog No.:BCN7298
CAS No.:121587-18-6
- 6-Demethoxycleomiscosin A
Catalog No.:BCN7299
CAS No.:121587-20-0
- YM 298198 hydrochloride
Catalog No.:BCC7366
CAS No.:1216398-09-2
- SB 258585 hydrochloride
Catalog No.:BCC7216
CAS No.:1216468-02-8
- Kaempferol-3-O-(2',6'-di-O-trans-p-coumaroyl)-beta-D-glucopyranoside
Catalog No.:BCN1603
CAS No.:121651-61-4
- BX 513 hydrochloride
Catalog No.:BCC5940
CAS No.:1216540-18-9
- ZK 93423 hydrochloride
Catalog No.:BCC7227
CAS No.:1216574-52-5
- 2-Cyclopropyl-4-(4-fluorophenyl)-quinolyl-3-methanol
Catalog No.:BCC8574
CAS No.:121660-11-5
- 2-Cyclopropyl-4-(4-fluorophenyl)quinoline-3-carboxaldehyde
Catalog No.:BCC8573
CAS No.:121660-37-5
- Trap 101
Catalog No.:BCC7390
CAS No.:1216621-00-9
Simultaneous determination of gatifloxacin and ambroxol hydrochloride from tablet dosage form using reversed-phase high performance liquid chromatography.[Pubmed:18724676]
Se Pu. 2008 May;26(3):358-61.
A reversed-phase high performance liquid chromatography (HPLC) method was developed, validated, and used for the quantitative determination of gatifloxacin (GA) and ambroxol hydrochloride (AM), from its tablet dosage form. Chromatographic separation was performed on a HiQ Sil C18 column (250 mm x 4.6 mm, 5 microm), with a mobile phase comprising of a mixture of 0.01 mol/L potassium dihydrogen orthophosphate buffer and acetonitrile (70 : 30, v/v), and pH adjusted to 3 with orthophosphoric acid, at a flow rate of 1 mL/min, with detection at 247 nm. Separation was completed in less than 10 min. As per International Conference on Harmonisation (ICH) guidelines the method was validated for linearity, accuracy, precision, limit of quantitation, limit of detection, and robustness. Linearity of GA was found to be in the range of 10 -60 microg/mL and that for AM was found to be 5 - 30 microg/mL. The correlation coefficients were 0.999 6 and 0.999 3 for GA and AM respectively. The results of the tablet analysis (n = 5) were found to be 99.94% with +/- 0.25% standard deviation (SD) and 99.98% with +/- 0.36% SD for GA and AM respectively. Percent recovery of GA was found to be 99.92% - 100.02% and that of AM was 99.86% - 100.16%. The assay experiment shows that the method is free from interference of excipients. This demonstrates that the developed HPLC method is simple, linear, precise, and accurate, and can be conveniently adopted for the routine quality control analysis of the tablet.
Evaluation of 0.3% gatifloxacin hydrochloride in decontamination of donor corneas.[Pubmed:22878383]
Eye Contact Lens. 2012 Sep;38(5):295-9.
PURPOSE: To evaluate demographic, clinical, and microbiological profile of eye donors and efficacy of 0.3% Gatifloxacin hydrochloride in microbial decontamination of donor corneas. METHODS: About 513 donors and 1,026 corneas received at National Eye Bank of a tertiary care hospital during 1-year period were analyzed prospectively in this randomized clinical trial. The donor eyes were graded and treated with 5% povidone-iodine, 0.4% amikacin sulphate, and 0.3% Gatifloxacin hydrochloride. The parameters evaluated were death enucleation time (DET), grading of donor corneas, microbiological profile of culture organisms, and their sensitivity to various antibiotics. RESULTS: Mean DET was 6.29+/-5.7 hours. Forty one percent eyes were optical grade corneas and the majority of donors (38.5%) had accidental deaths. Good grade eyes were maximum with DET of <1 hour and were comparable between 0-6 hours and 6-12 hours. About 57.6% (591/1026) eyes were culture positive; most common organisms were Pseudomonas spp (53%) and Coagulase-negative Staphylococci (24%). Culture positivity reduced significantly after treatment with povidone iodine and amikacin (P=0.002, right eye; P=0.004; left eye) and decreased further with use of gatifloxacin (P=0.001). Pseudomonas (93%), Coagulase-negative Staphylococci (96.3%), Staphylococcus aureus (90.5%), enterococci and gram-negative bacilli were sensitive to gatifloxacin. Pseudomonas spp which were multidrug-resistant were sensitive to polymyxin-B. CONCLUSIONS: Gatifloxacin hydrochloride in addition to amikacin sulphate is beneficial for donor eye decontamination. Polymyxin-B may be used for multidrug-resistant Pseudomonas spp.


