ZK 93423 hydrochlorideGABAA receptor agonist CAS# 1216574-52-5 |
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- Go 6976
Catalog No.:BCC3703
CAS No.:136194-77-9
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Chelerythrine chloride
Catalog No.:BCN8322
CAS No.:3895-92-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1216574-52-5 | SDF | Download SDF |
| PubChem ID | 56972166 | Appearance | Powder |
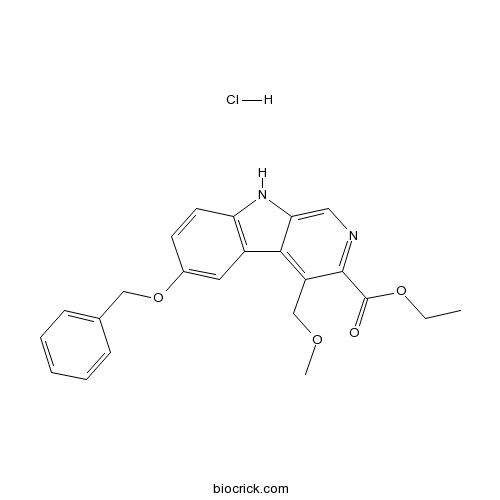
| Formula | C23H23ClN2O4 | M.Wt | 426.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 30 mM in DMSO and to 5 mM in ethanol | ||
| Chemical Name | ethyl 4-(methoxymethyl)-6-phenylmethoxy-9H-pyrido[3,4-b]indole-3-carboxylate;hydrochloride | ||
| SMILES | CCOC(=O)C1=NC=C2C(=C1COC)C3=C(N2)C=CC(=C3)OCC4=CC=CC=C4.Cl | ||
| Standard InChIKey | KHMDBPAODAAMBQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H22N2O4.ClH/c1-3-28-23(26)22-18(14-27-2)21-17-11-16(29-13-15-7-5-4-6-8-15)9-10-19(17)25-20(21)12-24-22;/h4-12,25H,3,13-14H2,1-2H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent benzodiazepine receptor agonist (IC50 = 1 nM). Non-selective between α1-, α2-, α3- and α5-subunit containing GABAA receptors (Ki values are 4.1, 4.2, 6 and 4.5 nM for inhibition of [3H]Ro15-1788 binding to human recombinant α1β3γ2, α2β3γ2, α3β3γ2 and α5β3γ2 receptors respectively). Anxiolytic following systemic administration in vivo. |

ZK 93423 hydrochloride Dilution Calculator

ZK 93423 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3425 mL | 11.7123 mL | 23.4247 mL | 46.8494 mL | 58.5617 mL |
| 5 mM | 0.4685 mL | 2.3425 mL | 4.6849 mL | 9.3699 mL | 11.7123 mL |
| 10 mM | 0.2342 mL | 1.1712 mL | 2.3425 mL | 4.6849 mL | 5.8562 mL |
| 50 mM | 0.0468 mL | 0.2342 mL | 0.4685 mL | 0.937 mL | 1.1712 mL |
| 100 mM | 0.0234 mL | 0.1171 mL | 0.2342 mL | 0.4685 mL | 0.5856 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 1 nM
ZK-93423 is an anxiolytic drug from the β-Carboline family, which is a nonbenzodiazepine GABAA receptor agonist. The GABAA receptor is an ligand-gated ion channel and ionotropic receptor.
In vitro: The full agonist ZK-93423 is not subtype selective and stimulates α1, α2, α3, and α5-subunit containing GABAA receptors equally [α1β32 (Ki = 4.1 nM), α2β32 (Ki = 4.2 nM), α3β32 (Ki = 6.0 nM), α5β32 (Ki = 4.5 nM), α6β32 (Ki >1000 nM)] [1]. ZK-93423 has also been used as a base to develop new and improved beta-carboline derivatives and help map the binding site of the GABAA receptor. In rats trained to discriminate PTZ from saline, the PTZ cue was antagonized by ZK 93423, which indicated ZK 93423 may exhibit anxiolytic quality [2].
In vivo: ZK 93423 showd to have anxiolytic properties on animal models of anxiety, including social interaction, vogel, geller serfter, 4-plate test and elevated plus-maze. The anxiogenic and anxiolytic actions are mediated by the BDZ binding sites [3].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Cox ED, Diaz-Arauzo H, Huang Q, Reddy MS, Ma C, Harris B, McKernan R, Skolnick P, Cook JM. Synthesis and evaluation of analogues of the partial agonist 6-(propyloxy)-4-(methoxymethyl)-beta-carboline-3-carboxylic acid ethyl ester (6-PBC) and the full agonist 6-(benzyloxy)-4-(methoxymethyl)-beta-carboline-3-carboxylic acid ethyl ester (Zk 93423) at wild type and recombinant GABAA receptors. J Med Chem. 1998 Jul 2;41(14):2537-52.
[2] Stephens DN, Shearman GT, Kehr W. Discriminative stimulus properties of beta-carbolines characterized as agonists and inverse agonists at central benzodiazepine receptors. Psychopharmacology (Berl). 1984;83(3):233-9.
[3] File SE, Baldwin HA. Effects of beta-carbolines in animal models of anxiety. Brain Res Bull. 1987 Sep;19(3):293-9.
- BX 513 hydrochloride
Catalog No.:BCC5940
CAS No.:1216540-18-9
- Kaempferol-3-O-(2',6'-di-O-trans-p-coumaroyl)-beta-D-glucopyranoside
Catalog No.:BCN1603
CAS No.:121651-61-4
- SB 258585 hydrochloride
Catalog No.:BCC7216
CAS No.:1216468-02-8
- YM 298198 hydrochloride
Catalog No.:BCC7366
CAS No.:1216398-09-2
- 6-Demethoxycleomiscosin A
Catalog No.:BCN7299
CAS No.:121587-20-0
- 6-Demethoxy-9'-deoxycleomiscosin A
Catalog No.:BCN7298
CAS No.:121587-18-6
- Valspodar
Catalog No.:BCC2027
CAS No.:121584-18-7
- DMCM hydrochloride
Catalog No.:BCC7560
CAS No.:1215833-62-7
- Gatifloxacin hydrochloride
Catalog No.:BCC4224
CAS No.:121577-32-0
- NBI 27914 hydrochloride
Catalog No.:BCC7124
CAS No.:1215766-76-9
- CP-809101 hydrochloride
Catalog No.:BCC1499
CAS No.:1215721-40-6
- RS 100329 hydrochloride
Catalog No.:BCC5741
CAS No.:1215654-26-4
- 2-Cyclopropyl-4-(4-fluorophenyl)-quinolyl-3-methanol
Catalog No.:BCC8574
CAS No.:121660-11-5
- 2-Cyclopropyl-4-(4-fluorophenyl)quinoline-3-carboxaldehyde
Catalog No.:BCC8573
CAS No.:121660-37-5
- Trap 101
Catalog No.:BCC7390
CAS No.:1216621-00-9
- SCH 79797 dihydrochloride
Catalog No.:BCC7125
CAS No.:1216720-69-2
- BYK 191023 dihydrochloride
Catalog No.:BCC7506
CAS No.:1216722-25-6
- GSK 4112
Catalog No.:BCC7741
CAS No.:1216744-19-2
- ZK 93426 hydrochloride
Catalog No.:BCC7229
CAS No.:1216792-30-1
- CGP 20712 dihydrochloride
Catalog No.:BCC6893
CAS No.:1216905-73-5
- Sarafotoxin S6c
Catalog No.:BCC5721
CAS No.:121695-87-2
- Moluccanin
Catalog No.:BCN6107
CAS No.:121700-26-3
- Moluccanin diacetate
Catalog No.:BCN6108
CAS No.:121700-27-4
- PTC209 HBr
Catalog No.:BCC5640
CAS No.:1217022-63-3
Synthesis and evaluation of analogues of the partial agonist 6-(propyloxy)-4-(methoxymethyl)-beta-carboline-3-carboxylic acid ethyl ester (6-PBC) and the full agonist 6-(benzyloxy)-4-(methoxymethyl)-beta-carboline-3-carboxylic acid ethyl ester (Zk 93423) at wild type and recombinant GABAA receptors.[Pubmed:9651158]
J Med Chem. 1998 Jul 2;41(14):2537-52.
A pharmacophore and an alignment rule have previously been reported for BzR agonist ligands. The design and synthesis of 6-(propyloxy)-4-(methoxymethyl)-beta-carboline-3-carboxylic acid ethyl ester (6-PBC, 24, IC50 = 8.1 nM) was based on this pharmacophore. When evaluated in vivo this ligand exhibited anticonvulsant/anxiolytic activity but was devoid of the muscle relaxant/ataxic effects of "classical" 1,4-benzodiazepines (i.e., diazepam). Significantly, 6-PBC 24 also reversed diazepam-induced muscle relaxation in mice. The 3-substituted analogues 40-46 and 48 of 6-PBC 24 and Zk 93423 27(IC50 = 1 nM) were synthesized and evaluated in vitro to determine what affect these modifications would have on the binding affinity at recombinant BzR subtypes. With the exception of the 3-amino ligands 40 and 41, all the beta-carbolines were found to exhibit high binding affinity at BzR sites. The 3-propyl ether derivative 45 was also evaluated in vivo and found to be devoid of any proconvulsant or anticonvulsant activity at doses up to 40 mg/kg. The 6-(1-naphthylmethyloxy) and 6-octyloxy analogues 25, 26, 28, and 29 of 6-PBC 24 were synthesized to further evaluate the proposed alignment of agonists vs inverse agonists in the pharmacophore of the BzR. In addition, ligands 26 and 29 were designed to probe the dimensions of lipophilic pocket L3 at the agonist site. The activity of 29 was evaluated in vivo; however, this analogue elicited no pharmacological effects at doses up to 80 mg/kg. These and other related beta-carbolines were also examined in five recombinant GABAA receptor subtypes. Ligands 52-61 all exhibited moderate to high affinity at GABAA receptors containing alpha1 subunits. These ligands will be useful in further defining the pharmacophore at alpha1 beta3 gamma2 receptors.
Effects of beta-carbolines in animal models of anxiety.[Pubmed:3315125]
Brain Res Bull. 1987 Sep;19(3):293-9.
Animal models of anxiety can be classified into three main groups: those based on conflict or conditioned fear; those exploiting the anxiety produced by novelty; those in which anxiety or aversion is chemically induced. This review briefly describes the existing tests and, where available, the results obtained with beta-carbolines. Many of the beta-carbolines are anxiogenic in the tests, however ZK 91296 and ZK 93423 appear to have anxiolytic properties, and ZK 93426 has a similar profile to that of the benzodiazepine receptor antagonist RO 15-1788. By the results across the spectrum of tests, the reliability and sensitivity of the tests is assessed. The evidence that the anxiogenic and anxiolytic actions of the beta-carbolines are mediated by the BDZ binding sites is also discussed.
Discriminative stimulus properties of beta-carbolines characterized as agonists and inverse agonists at central benzodiazepine receptors.[Pubmed:6089245]
Psychopharmacology (Berl). 1984;83(3):233-9.
The discriminative stimulus properties of three beta-carboline derivatives were studied in three groups of rats trained, respectively, to discriminate diazepam (2.5 mg/kg IP), chlordiazepoxide (CDP, 5 mg/kg IP) or pentylenetetrazol (PTZ, 15 mg/kg IP) from saline in standard procedures employing two-lever operant chambers. Two beta-carbolines, ZK 91296 and ZK 93423, substituted for the benzodiazepines in both CDP- and diazepam-trained rats. The neutral benzodiazepine antagonists Ro 15-1788 blocked the diazepam discriminative stimulus and the ability of ZK 91296 to substitute for diazepam. A third beta-carboline, FG 7142, was not identified as benzodiazepine-like in generalization tests in either diazepam- or CDP-trained rats, but when administered together with CDP antagonized the benzodiazepine discriminative stimulus. In rats trained to discriminate PTZ from saline (a discrimination which is thought to depend on the anxiogenic properties of PTZ) the PTZ cue was antagonized by diazepam and ZK 93423, and partially antagonized by ZK 91296. The PTZ cue generalized to FG 7142 and this generalization was partially antagonized by Ro 15-1788. These results suggest that the three beta-carbolines provide more than one kind of discriminative stimulus, consistent with the classification of ZK 93423 as an agonist at central benzodiazepine receptors, with ZK 91296 as a partial agonist, and with FG 7142 as an inverse agonist. Pharmacologically, ZK 93423 and ZK 91296 may exhibit anxiolytic qualities, whereas FG 7142 produces anxiogenic effects.


