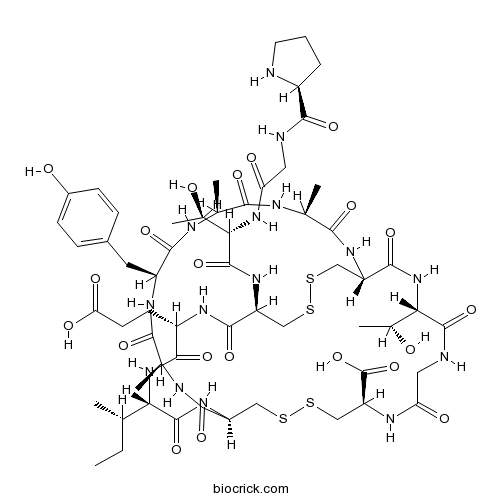
Guanylin (human)Endogenous activator of intestinal guanylyl cyclase CAS# 183200-12-6 |
- Salmefamol
Catalog No.:BCC1919
CAS No.:18910-65-1
- (R,R)-Formoterol
Catalog No.:BCC1293
CAS No.:67346-49-0
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
- Medetomidine
Catalog No.:BCC1736
CAS No.:86347-14-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 183200-12-6 | SDF | Download SDF |
| PubChem ID | 90488722 | Appearance | Powder |
| Formula | C58H87N15O21S4 | M.Wt | 1458.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in water | ||
| Sequence | PGTCEICAYAACTGC (Modifications: Cys-15 = C-terminal OH, Disulfide Bridge between 4 - 2, 7 - 5) | ||
| Chemical Name | (1R,4S,7S,10S,13S,16R,21R,27S,34R,37S,40S)-40-[(2S)-butan-2-yl]-37-(2-carboxyethyl)-27-[(1R)-1-hydroxyethyl]-10-[(4-hydroxyphenyl)methyl]-34-[[(2S,3R)-3-hydroxy-2-[[2-[[(2S)-pyrrolidine-2-carbonyl]amino]acetyl]amino]butanoyl]amino]-4,7,13-trimethyl-3,6,9,12,15,23,26,29,35,38,41-undecaoxo-18,19,31,32-tetrathia-2,5,8,11,14,22,25,28,36,39,42-undecazabicyclo[14.13.13]dotetracontane-21-carboxylic acid | ||
| SMILES | CCC(C)C1C(=O)NC2CSSCC(NC(=O)CNC(=O)C(NC(=O)C(CSSCC(C(=O)NC(C(=O)N1)CCC(=O)O)NC(=O)C(C(C)O)NC(=O)CNC(=O)C3CCCN3)NC(=O)C(NC(=O)C(NC(=O)C(NC(=O)C(NC2=O)C)CC4=CC=C(C=C4)O)C)C)C(C)O)C(=O)O | ||
| Standard InChIKey | XPNQMTAYRNMRRD-RDJGHUJSSA-N | ||
| Standard InChI | InChI=1S/C58H87N15O21S4/c1-8-25(2)43-56(91)69-36-21-97-98-24-39(58(93)94)65-40(77)19-61-55(90)44(29(6)74)73-54(89)38(68-48(83)27(4)62-46(81)26(3)63-51(86)35(18-31-11-13-32(76)14-12-31)67-47(82)28(5)64-52(36)87)23-96-95-22-37(53(88)66-34(50(85)72-43)15-16-42(79)80)70-57(92)45(30(7)75)71-41(78)20-60-49(84)33-10-9-17-59-33/h11-14,25-30,33-39,43-45,59,74-76H,8-10,15-24H2,1-7H3,(H,60,84)(H,61,90)(H,62,81)(H,63,86)(H,64,87)(H,65,77)(H,66,88)(H,67,82)(H,68,83)(H,69,91)(H,70,92)(H,71,78)(H,72,85)(H,73,89)(H,79,80)(H,93,94)/t25-,26-,27-,28-,29+,30+,33-,34-,35-,36-,37-,38-,39-,43-,44-,45-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous peptide activator of intestinal guanylyl cyclase; secreted mainly by the gastrointestinal mucosa. Regulates water and electrolyte transport in intestinal and renal epithelia. |

Guanylin (human) Dilution Calculator

Guanylin (human) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cabazitaxel
Catalog No.:BCC4966
CAS No.:183133-96-2
- Fabiatrin
Catalog No.:BCN2920
CAS No.:18309-73-4
- 3,4-O-Isopropylidene shikimic acid
Catalog No.:BCN1147
CAS No.:183075-03-8
- Didrovaltrate
Catalog No.:BCN7124
CAS No.:18296-45-2
- Valepotriate
Catalog No.:BCN2351
CAS No.:18296-44-1
- Dibritannilactone B
Catalog No.:BCN7776
CAS No.:1829580-18-8
- (9Z,12Z)-N-Benzyloctadeca-9,12-dienamide
Catalog No.:BCN1518
CAS No.:18286-71-0
- Thalidezine
Catalog No.:BCN7763
CAS No.:18251-36-0
- SB 225002
Catalog No.:BCC8077
CAS No.:182498-32-4
- TPMPA
Catalog No.:BCC6903
CAS No.:182485-36-5
- Lomitapide
Catalog No.:BCC5570
CAS No.:182431-12-5
- Rupatadine Fumarate
Catalog No.:BCC4535
CAS No.:182349-12-8
- Tipiracil hydrochloride
Catalog No.:BCC2001
CAS No.:183204-72-0
- 1,7-Dihydroxy-2,3-methylenedioxyxanthone
Catalog No.:BCN7543
CAS No.:183210-63-1
- AM251
Catalog No.:BCC4412
CAS No.:183232-66-8
- 2-Acetoxy-3-deacetoxycaesaldekarin E
Catalog No.:BCN7476
CAS No.:18326-06-2
- 5-(1-Piperazinyl)benzofuran-2-carboxamide
Catalog No.:BCC8717
CAS No.:183288-46-2
- Erlotinib Hydrochloride
Catalog No.:BCC3645
CAS No.:183319-69-9
- OSI-420
Catalog No.:BCC4472
CAS No.:183320-51-6
- Erlotinib
Catalog No.:BCC1557
CAS No.:183321-74-6
- CPPG
Catalog No.:BCC6872
CAS No.:183364-82-1
- Apicidin
Catalog No.:BCC3599
CAS No.:183506-66-3
- Cleroindicin A
Catalog No.:BCC8916
CAS No.:176598-06-4
- CYN 154806
Catalog No.:BCC5823
CAS No.:183658-72-2
Guanylin and E. coli heat-stable enterotoxin induce chloride secretion through direct interaction with basolateral compartment of rat and human colonic cells.[Pubmed:15901896]
Pediatr Res. 2005 Jul;58(1):159-63.
We previously detected specific binding activity of Escherichia coli heat-stable enterotoxin (ST), the guanylin exogenous ligand, in rat colonic basolateral membranes. Because guanylin circulates in the bloodstream, we tested the hypothesis that it modulates intestinal ion transport by acting on the serosal side of intestinal cells. The effects of the mucosal and serosal addition of ST and guanylin on ion transport were investigated in the rat proximal colon and in Caco-2 cells in Ussing chambers, by monitoring short-circuit current (Isc). cGMP concentration was measured in Caco-2 cells by RIA. Mucosal ST addition induced an increase in Isc in rat proximal colon consistent with anion secretion. Serosal addition induced the same effects but to a lesser extent. The electrical effects observed in Caco-2 cells paralleled those observed in rat proximal colon. A pattern similar to the electrical response was observed with cGMP concentration. Guanylin addition to either side of Caco-2 cells induced the same effects as ST, although to a lesser extent. In all conditions, the electrical effect disappeared in the absence of chloride. ST directly interacts with basolateral receptors in the large intestine inducing chloride secretion through an increase of cGMP. However, the serosal effects are less pronounced compared with those observed with mucosal addition. Guanylin shows the same pattern, suggesting that it plays a role in the regulation of ion transport in the colon, but the relative importance of serosally mediated secretion remains to be determined.
Guanylin and uroguanylin stimulate lipolysis in human visceral adipocytes.[Pubmed:27108812]
Int J Obes (Lond). 2016 Sep;40(9):1405-15.
BACKGROUND/OBJECTIVES: Uroguanylin and guanylin are secreted by intestinal epithelial cells as prohormones postprandially and act on the hypothalamus to induce satiety. The impact of obesity and obesity-associated type 2 diabetes (T2D) on proguanylin and prouroguanylin expression/secretion as well as the potential role of guanylin and uroguanylin in the control of lipolysis in humans was evaluated. SUBJECTS/METHODS: Circulating and gastrointestinal expression of proguanylin (GUCA2A) and prouroguanylin (GUCA2B) were measured in 134 subjects. In addition, plasma proguanylin and prouroguanylin were measured before and after weight loss achieved either by Roux-en-Y gastric bypass (RYGB) (n=24) or after a conventional diet (n=15). The effect of guanylin and uroguanylin (1-100 nmol l(-1)) on lipolysis was determined in vitro in omental adipocytes. RESULTS: Circulating concentrations of prouroguanylin, but not proguanylin, were decreased in obesity in relation to adiposity. Weight loss achieved by RYGB increased plasma proguanylin and prouroguanylin. Obese T2D individuals showed higher expression of intestinal GUCA2A as well as of the receptors of the guanylin system, GUCY2C and GUCY2D, in omental adipocytes. The incubation with guanylin and uroguanylin significantly stimulated lipolysis in differentiated omental adipocytes, as evidenced by hormone-sensitive lipase phosphorylation at Ser563, an increase in fatty acids and glycerol release together with an upregulation of several lipolysis-related genes, including AQP3, AQP7, FATP1 or CD36. CONCLUSIONS: Both guanylin and uroguanylin trigger lipolysis in human visceral adipocytes. Given the lipolytic action of the guanylin system on visceral adipocytes, the herein reported decrease of circulating prouroguanylin concentrations in obese patients may have a role in excessive fat accumulation in obesity.
Cellular localization of guanylin and uroguanylin mRNAs in human and rat duodenal and colonic mucosa.[Pubmed:27044258]
Cell Tissue Res. 2016 Aug;365(2):331-41.
Guanylin (GUCA2A/Guca2a/GN) and uroguanylin (GUCA2B/Guca2b/UGN) are expressed in the gastrointestinal tract and have been implicated in ion and fluid homeostasis, satiety, abdominal pain, growth and intestinal barrier integrity. Their cellular sources are debated and include goblet cells, entero-/colonocytes, enteroendocrine (EE) cells and tuft cells. We therefore investigated the cellular sources of GN and UGN mRNAs in human and rat duodenal and colonic epithelium with in situ hybridization (ISH) to determine co-expression with Chromogranin A (CHGA/Chga/CgA; enterochromaffin [EC] cells), defensin alpha 6 (DEFA6/Defa6; Paneth cells), mucin 2 (MUC2/Muc2; goblet cells) and selected tuft cell markers. GUCA2A/Guca2a expression was localized to goblet cells and colonocytes in human and rat colon. In human duodenum, GUCA2A was expressed in Paneth cells and was scarce in villous epithelial cells. In rat duodenum, Guca2a was only localized to goblet cells. Guca2b was focally expressed in rat colon. In human and rat duodenum and in human colon, GUCA2B/Guca2b was expressed in dispersed solitary epithelial cells, some with a tuft cell-like appearance. Neither GUCA2A nor GUCA2B were co-expressed with CHGA in human duodenal cells. Consequently, EC cells are probably not the major source of human GN or UGN but other EE cells as a source of GN or UGN are not entirely excluded. No convincing overlap with tuft cell markers was found. For the first time, we demonstrate the cellular expression of GUCA2B in human duodenum. The specific cellular distribution of both GN and UGN differs between duodenum and colon and between human and rat intestines.
Characterization of immunological cross-reactivity between enterotoxigenic Escherichia coli heat-stable toxin and human guanylin and uroguanylin.[Pubmed:24778111]
Infect Immun. 2014 Jul;82(7):2913-22.
Enterotoxigenic Escherichia coli (ETEC) expressing the heat-stable toxin (ST) (human-type [STh] and porcine-type [STp] variants) is among the five most important enteric pathogens in young children living in low- and middle-income countries. ST mediates diarrheal disease through activation of the guanylate cyclase C (GC-C) receptor and is an attractive vaccine target with the potential to confer protection against a wide range of ETEC strains. However, immunological cross-reactivity to the endogenous GC-C ligands guanylin and uroguanylin is a major concern because of the similarities to ST in amino acid sequence, structure, and function. We have investigated the presence of similar epitopes on STh, STp, guanylin, and uroguanylin by analyzing these peptides in eight distinct competitive enzyme-linked immunosorbent assays (ELISAs). A fraction (27%) of a polyclonal anti-STh antibody and an anti-STh monoclonal antibody (MAb) cross-reacted with uroguanylin, the latter with a 73-fold-lower affinity. In contrast, none of the antibodies raised against STp, one polyclonal antibody and three MAbs, cross-reacted with the endogenous peptides. Antibodies raised against guanylin and uroguanylin showed partial cross-reactivity with the ST peptides. Our results demonstrate, for the first time, that immunological cross-reactions between ST and the endogenous peptides can occur. However, the partial nature and low affinity of the observed cross-reactions suggest that the risk of adverse effects from a future ST vaccine may be low. Furthermore, our results suggest that this risk may be reduced or eliminated by basing an ST immunogen on STp or a selectively mutated variant of STh.
Guanylin and related peptides.[Pubmed:11596856]
J Physiol Pharmacol. 2001 Sep;52(3):351-75.
Guanylin and uroguanylin are short peptides homologous to heat-stable enterotoxins of Escherichia coli and other enteric bacteria. Guanylin and uroguanylin are synthetized from the respective prepropeptides mainly in gastrointestinal mucosa and are secreted both into intestinal lumen and into the blood. Luminally secreted peptides stimulate chloride and bicarbonate secretion in the intestine through the mechanism involving guanylate cyclase C receptor, cyclic GMP, protein kinase G and cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel. Bacterial enterotoxins, which have greater potency than endogenous peptides, induce excessive fluid secretion into intestinal lumen leading to secretory diarhea. Uroguanylin is expressed mainly in enterochromaffin cells of duodenum and proximal small intestine whereas guanylin is abundant in goblet cells of colonic epithelium. Uroguanylin and guanylin increase urinary sodium and potassium excretion both as circulating hormones and as paracrine mediators produced within the kidney. Uroguanylin functions as "intestinal natriuretic hormone" which is secreted in response to oral sodium loading and maintains sodium balance during postprandial period. Plasma and urinary concentrations of guanylin and uroguanylin increase in renal failure and heart failure. Guanylin peptides possess antiproliferative activity in intestinal cells culture and their expression decreases in colonic carcinoma indicating that their deficiency may contribute to the pathogenesis of this disease.
Synthesis, solution structure, binding activity, and cGMP activation of human guanylin and its disulfide isomer.[Pubmed:9272623]
Regul Pept. 1997 Jun 18;70(2-3):111-20.
Guanylin is a recently isolated peptide consisting of 15 amino acid residues with four cysteines, which may form two intramolecular disulfide bridges, and stimulates intestinal membrane guanylate cyclase. The position of the disulfide linkages of guanylin was predicted from its structural similarity to a heat stable enterotoxin which is thought to be responsible for secretory diarrhoea. Both guanylin, with disulfide positions 4-12 and 7-15, and its disulfide isomer, with disulfides positions 4-15 and 7-12, were chemically synthesized by the solid-phase method and purified. Two specific disulfides were selectively formed and confirmed by sequencing, mass spectrometry and high-performance liquid chromatography in combination with enzymatic cleavage. The structure of both isomers has been investigated in solution by 1H nuclear magnetic resonance spectroscopy. Guanylin exists as a mixture of two stable conformations which have compact spiral structures, from comparison with literature data. In contrast, the disulfide isomer of guanylin shows only a single conformation with an elongated curved plate-like structure. Binding assays were performed using labelled guanylin with membranes obtained from rat jejunum. Both disulfide isomers were investigated by the cGMP assay. Both binding and cGMP assays indicated that the relevant form of disulfide bridges in the intact guanylin was as predicted.
Guanylin: an endogenous activator of intestinal guanylate cyclase.[Pubmed:1346555]
Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):947-51.
Intestinal guanylate cyclase mediates the action of the heat-stable enterotoxin to cause a decrease in intestinal fluid absorption and to increase chloride secretion, ultimately causing diarrhea. An endogenous ligand that acts on this guanylate cyclase has not previously been found. To search for a potential endogenous ligand, we utilized T84 cells, a human colon carcinoma-derived cell line, in culture as a bioassay. This cell line selectively responds to the toxin in a very sensitive manner with an increase in intracellular cyclic GMP. In the present study, we describe the purification and structure of a peptide from rat jejunum that activates this enzyme. This peptide, which we have termed guanylin, is composed of 15 amino acids and has the following amino acid sequence, PNTCEICAYAACTGC, as determined by automated Edman degradation sequence analysis and electrospray mass spectrometry. Analysis of the amino acid sequence of this peptide reveals a high degree of homology with heat-stable enterotoxins. Solid-phase synthesis of this peptide confirmed that it stimulates increases in T84 cyclic GMP levels. Guanylin required oxidation for expression of bioactivity and subsequent reduction of the oxidized peptide eliminated the effect on cyclic GMP, indicating a requirement for cysteine disulfide bond formation. Synthetic guanylin also displaces heat-stable enterotoxin binding to cultured T84 cells. Based on these data, we propose that guanylin is an activator of intestinal guanylate cyclase and that it stimulates this enzyme through the same receptor binding region as the heat-stable enterotoxins.


