OSI-420EGFR inhibitor CAS# 183320-51-6 |
- Zanamivir
Catalog No.:BCC4946
CAS No.:139110-80-8
- Oseltamivir acid
Catalog No.:BCC1826
CAS No.:187227-45-8
- Oseltamivir phosphate
Catalog No.:BCC4690
CAS No.:204255-11-8
- Peramivir
Catalog No.:BCC1846
CAS No.:330600-85-6
- Nucleozin
Catalog No.:BCC1811
CAS No.:341001-38-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 183320-51-6 | SDF | Download SDF |
| PubChem ID | 18924996 | Appearance | Powder |
| Formula | C21H22ClN3O4 | M.Wt | 415.87 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Desmethyl Erlotinib; CP-473420 | ||
| Solubility | DMSO : 50 mg/mL (120.23 mM; Need ultrasonic) | ||
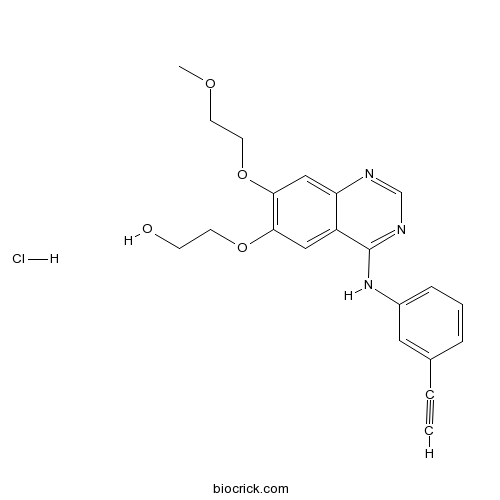
| Chemical Name | 2-[4-(3-ethynylanilino)-7-(2-methoxyethoxy)quinazolin-6-yl]oxyethanol;hydrochloride | ||
| SMILES | COCCOC1=C(C=C2C(=C1)N=CN=C2NC3=CC=CC(=C3)C#C)OCCO.Cl | ||
| Standard InChIKey | BUOXOWNQZVIETJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H21N3O4.ClH/c1-3-15-5-4-6-16(11-15)24-21-17-12-19(27-8-7-25)20(28-10-9-26-2)13-18(17)22-14-23-21;/h1,4-6,11-14,25H,7-10H2,2H3,(H,22,23,24);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | OSI-420 is the active metabolite of Erlotinib (EGFR inhibitor with IC50 of 2 nM). | |||||
| Targets | EGFR | |||||

OSI-420 Dilution Calculator

OSI-420 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4046 mL | 12.023 mL | 24.046 mL | 48.092 mL | 60.1149 mL |
| 5 mM | 0.4809 mL | 2.4046 mL | 4.8092 mL | 9.6184 mL | 12.023 mL |
| 10 mM | 0.2405 mL | 1.2023 mL | 2.4046 mL | 4.8092 mL | 6.0115 mL |
| 50 mM | 0.0481 mL | 0.2405 mL | 0.4809 mL | 0.9618 mL | 1.2023 mL |
| 100 mM | 0.024 mL | 0.1202 mL | 0.2405 mL | 0.4809 mL | 0.6011 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
OSI-420 is the major metabolite of OSI-774(erlotinib) which is a small molecule, orally active, potent, and selective inhibitor of EGFR tyrosine kinase with an IC50 value of 2nM[1,2].
OSI-420 is the active metabolite of OSI-774(erlotinib) which selectively inhibits the EGFR tyrosine kinase and prevents autophosphorylation by competing with adenosine triphosphate (ATP) for its binding site on the intracellular domain of EGFR. In addition, erlotinib, is metabolized to produce ATP, has been found to be selective for EGFR and lead to the induction of apoptosis by inducing the disruption of mitochondrial effect on loss of mitochondrial membrane potential and release of cytocrome c [1, 2].
References:
[1] Zhang W1, Siu LL, Moore MJ, Chen EX.Simultaneous determination of OSI-774 and its major metabolite OSI-420 in human plasma by using HPLC with UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Jan 5;814(1):143-7.
[2] Bonomi P. Erlotinib: a new therapeutic approach for non-small cell lung cancer.Expert Opin Investig Drugs. 2003 Aug;12(8):1395-401.
- Erlotinib Hydrochloride
Catalog No.:BCC3645
CAS No.:183319-69-9
- 5-(1-Piperazinyl)benzofuran-2-carboxamide
Catalog No.:BCC8717
CAS No.:183288-46-2
- 2-Acetoxy-3-deacetoxycaesaldekarin E
Catalog No.:BCN7476
CAS No.:18326-06-2
- AM251
Catalog No.:BCC4412
CAS No.:183232-66-8
- 1,7-Dihydroxy-2,3-methylenedioxyxanthone
Catalog No.:BCN7543
CAS No.:183210-63-1
- Tipiracil hydrochloride
Catalog No.:BCC2001
CAS No.:183204-72-0
- Guanylin (human)
Catalog No.:BCC7204
CAS No.:183200-12-6
- Cabazitaxel
Catalog No.:BCC4966
CAS No.:183133-96-2
- Fabiatrin
Catalog No.:BCN2920
CAS No.:18309-73-4
- 3,4-O-Isopropylidene shikimic acid
Catalog No.:BCN1147
CAS No.:183075-03-8
- Didrovaltrate
Catalog No.:BCN7124
CAS No.:18296-45-2
- Valepotriate
Catalog No.:BCN2351
CAS No.:18296-44-1
- Erlotinib
Catalog No.:BCC1557
CAS No.:183321-74-6
- CPPG
Catalog No.:BCC6872
CAS No.:183364-82-1
- Apicidin
Catalog No.:BCC3599
CAS No.:183506-66-3
- Cleroindicin A
Catalog No.:BCC8916
CAS No.:176598-06-4
- CYN 154806
Catalog No.:BCC5823
CAS No.:183658-72-2
- Penthiopyrad
Catalog No.:BCC8072
CAS No.:183675-82-3
- 1,2,3,4,5,6-Hexabromocyclohexane
Catalog No.:BCC2437
CAS No.:1837-91-8
- MRS 1220
Catalog No.:BCC6972
CAS No.:183721-15-5
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Cyanidin 3-sophoroside chloride
Catalog No.:BCN2611
CAS No.:18376-31-3
- Mithramycin A
Catalog No.:BCC2470
CAS No.:18378-89-7
- Ciproxifan
Catalog No.:BCC4539
CAS No.:184025-18-1
A validated liquid chromatography tandem mass spectrometry method for quantification of erlotinib, OSI-420 and didesmethyl erlotinib and semi-quantification of erlotinib metabolites in human plasma.[Pubmed:25594896]
J Pharm Biomed Anal. 2015 Mar 25;107:186-95.
A liquid chromatography tandem mass spectrometry method was developed and validated for quantification of erlotinib and its metabolites in human plasma. The method is suitable for therapeutic drug monitoring and pharmacokinetic studies. The substances were extracted using protein precipitation, separated on a BEH XBridge C18 column (100 x2.1 mm, 1.7 mum) by gradient elution at 0.7 mL/min of acetonitrile and 5 mM ammonium acetate. The concentration was determined using a Waters Xevo triple quadrupole mass spectrometer in a multi reaction monitoring mode. The total run time was 7 min. Deuterated erlotinib and OSI-597 were used as internal standard for erlotinib and its metabolites, respectively. Erlotinib, OSI-420 and didesmethyl erlotinib were quantified in the concentration range 25-5000 ng/mL, 0.5-500 ng/mL and 0.15-10 ng/mL, respectively. Precision and accuracy was <14% except for OSI-420 at LLOQ (17%). Extraction recovery was above 89%, 99% and 89% for erlotinib, OSI-420 and didesmethyl erlotinib, respectively. The human liver microsomes generated 14 metabolites, three of them not previously reported. Twelve metabolites were measured semi-quantitatively and validated with respect to selectivity, precision and stability.
Simultaneous Determination of Celecoxib, Erlotinib, and its Metabolite Desmethyl-Erlotinib (OSI-420) in Rat Plasma by Liquid chromatography/Tandem Mass Spectrometry with Positive/Negative Ion-Switching Electrospray Ionisation.[Pubmed:23008811]
Sci Pharm. 2012 Jul-Sep;80(3):633-46.
A new method for the simultaneous determination of celecoxib, erlotinib, and its active metabolite desmethyl-erlotinib (OSI-420) in rat plasma, by liquid chromatography/tandem mass spectrometry with positive/negative ion-switching electrospray ionization mode, was developed and validated. Protein precipitation with methanol was selected as the method for preparing the samples. The analytes were separated on a reverse-phase C(18) column (50mmx4.6mm i.d., 3mu) using methanol: 2 mM ammonium acetate buffer, and pH 4.0 as the mobile phase at a flow rate 0.8 mL/min. Sitagliptin and Efervirenz were used as the internal standards for quantification. The determination was carried out on a Theremo Finnigan Quantam ultra triple-quadrupole mass spectrometer, operated in selected reaction monitoring (SRM) mode using the following transitions monitored simultaneously: positive m/z 394.5-->278.1 for erlotinib, m/z 380.3-->278.1 for desmethyl erlotinib (OSI-420), and negative m/z -380.1--> -316.3 for celecoxib. The limits of quantification (LOQs) were 1.5 ng/mL for Celecoxib, erlotinib, and OSI-420. Within- and between-day accuracy and precision of the validated method were within the acceptable limits of < 15% at all concentrations. The quantitation method was successfully applied for the simultaneous estimation of celecoxib, erlotinib, and desmethyl erlotinib in a pharmacokinetic study in Wistar rats.
Plasma and pleural fluid pharmacokinetics of erlotinib and its active metabolite OSI-420 in patients with non-small-cell lung cancer with pleural effusion.[Pubmed:21775215]
Clin Lung Cancer. 2011 Sep;12(5):307-12.
BACKGROUND: Erlotinib is orally active and selectively inhibits the tyrosine kinase activity of the epidermal growth factor receptor. The pleural space penetration and exposure of erlotinib is poorly understood. Thus, we investigated the pharmacokinetics (PK) of erlotinib and its active metabolite OSI-420 in non-small-cell lung cancer (NSCLC) of malignant pleural effusion (MPE). PATIENTS AND METHODS: We analyzed the PK of erlotinib and OSI-420 on days 1 and 8 after beginning erlotinib therapy in 9 patients with MPE. Their concentrations were determined by high-performance liquid chromatography with ultraviolet detection. Blood samples were obtained five times per day: before administration, and 2, 4, 8, and 24 hours after administration. Pleural effusions were obtained once per day, 2 hours after administration on day 1, and before administration on day 8. The exceptions were cases 2 and 4, which had pleural effusions obtained just before drug administration, and 2, 4, 8, and 24 hours after administration. RESULTS: The mean percentage of penetration from plasma to pleural effusion for erlotinib was 18% on day 1 and 112% on day 8, while these values for OSI-420 were 9.5% on day 1 and 131% on day 8. The area under the drug concentration-time curve of pleural fluid for erlotinib was 28,406 ng-hr/mL for case 2 and 45,906 ng-hr/mL for case 4. CONCLUSIONS: There seems to be a significant accumulation of both erlotinib and OSI-420 in MPE with repeated dosing. Although larger studies will be necessary to determine the true impact of erlotinib MPE accumulation on plasma PK and safety, erlotinib can be administered safely to patients with MPE with respect to efficacy and side effects.
Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer.[Pubmed:20479691]
J Thorac Oncol. 2010 Jul;5(7):950-5.
BACKGROUND: Although there have been several reports in which central nervous system (CNS) metastases of non-small cell lung cancer (NSCLC) were improved by erlotinib, cerebrospinal fluid (CSF) penetration of erlotinib in such patients has not been reported. We investigated CSF concentrations of erlotinib and its active metabolite OSI-420. METHOD: We administered 150 mg erlotinib daily to four patients with NSCLC who had CNS metastases, and we investigated plasma pharmacokinetics of erlotinib and OSI-420 on days 1 and 8. In addition, we measured the concentrations of erlotinib and OSI-420 in CSF just before administration of erlotinib on day 8. RESULTS: In all cases except for one case, plasma pharmacokinetics data on day 8 were similar to those previously reported. The mean +/- SD CSF concentrations of erlotinib and OSI-420 were 54 +/- 30 ng/ml and 10.8 +/- 8.2 ng/ml, respectively. The mean +/- SD CSF penetration rates of erlotinib and OSI-420 were 5.1% +/- 1.9% and 5.8% +/- 3.6%, respectively. CSF concentrations of erlotinib exceeded median inhibitory concentration (IC50) of erlotinib in intact tumor cells with wild-type epidermal growth factor receptor gene. CONCLUSION: The CSF penetrations of erlotinib and OSI-420 in patients with NSCLC who had CNS metastases were approximately 5.1% and 5.8%, respectively. This indicates that erlotinib can become a treatment option for CNS metastases of NSCLC.


