HX 531Potent RXR antagonist CAS# 188844-34-0 |
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 188844-34-0 | SDF | Download SDF |
| PubChem ID | 11755040 | Appearance | Powder |
| Formula | C29H29N3O4 | M.Wt | 483.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in DMSO | ||
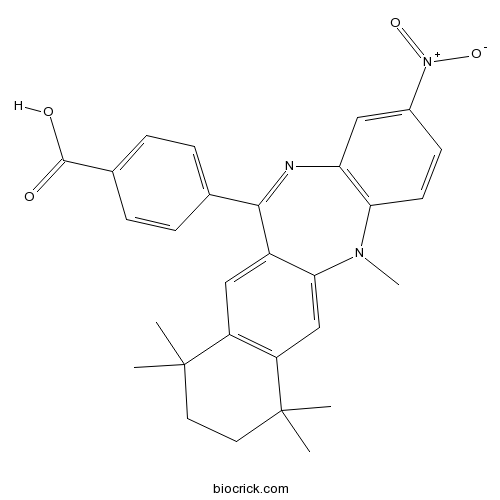
| Chemical Name | 4-(5,7,7,10,10-pentamethyl-2-nitro-8,9-dihydronaphtho[2,3-b][1,5]benzodiazepin-12-yl)benzoic acid | ||
| SMILES | CC1(CCC(C2=C1C=C3C(=C2)N(C4=C(C=C(C=C4)[N+](=O)[O-])N=C3C5=CC=C(C=C5)C(=O)O)C)(C)C)C | ||
| Standard InChIKey | SXKPGYKPQPYJER-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H29N3O4/c1-28(2)12-13-29(3,4)22-16-25-20(15-21(22)28)26(17-6-8-18(9-7-17)27(33)34)30-23-14-19(32(35)36)10-11-24(23)31(25)5/h6-11,14-16H,12-13H2,1-5H3,(H,33,34) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent RXR antagonist (IC50 = 18 nM). Promotes white and brown pre-adipocyte differentiation into white adipocytes. Also inhibits bexarotene-induced brown adipogenic reprogramming of myoblasts. |

HX 531 Dilution Calculator

HX 531 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.068 mL | 10.34 mL | 20.68 mL | 41.3599 mL | 51.6999 mL |
| 5 mM | 0.4136 mL | 2.068 mL | 4.136 mL | 8.272 mL | 10.34 mL |
| 10 mM | 0.2068 mL | 1.034 mL | 2.068 mL | 4.136 mL | 5.17 mL |
| 50 mM | 0.0414 mL | 0.2068 mL | 0.4136 mL | 0.8272 mL | 1.034 mL |
| 100 mM | 0.0207 mL | 0.1034 mL | 0.2068 mL | 0.4136 mL | 0.517 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Streptozotocin
Catalog No.:BCN3834
CAS No.:18883-66-4
- 8-Glucosyl-5,7-dihydroxy-2-(1-methylpropyl)chromone
Catalog No.:BCN7505
CAS No.:188818-27-1
- SC 560
Catalog No.:BCC7111
CAS No.:188817-13-2
- N-Acetylcaprolactam
Catalog No.:BCC9081
CAS No.:1888-91-1
- 4,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6492
CAS No.:188742-80-5
- 3-hydroxymorindone
Catalog No.:BCN3126
CAS No.:80368-74-7
- GSK3787
Catalog No.:BCC2263
CAS No.:188591-46-0
- Cl-4AS-1
Catalog No.:BCC7780
CAS No.:188589-66-4
- TFM-4AS-1
Catalog No.:BCC6069
CAS No.:188589-61-9
- SBI-0206965
Catalog No.:BCC3984
CAS No.:1884220-36-3
- Scandoside
Catalog No.:BCN3449
CAS No.:18842-99-4
- Paederosidic acid
Catalog No.:BCN3438
CAS No.:18842-98-3
- HX 630
Catalog No.:BCC6083
CAS No.:188844-52-2
- DMA
Catalog No.:BCC1532
CAS No.:188860-26-6
- Hydroxytanshinone IIA
Catalog No.:BCN2497
CAS No.:18887-18-8
- Methyl tanshinonate
Catalog No.:BCN2553
CAS No.:18887-19-9
- Junipediol B 8-O-glucoside
Catalog No.:BCN4022
CAS No.:188894-19-1
- L 760735
Catalog No.:BCC7840
CAS No.:188923-01-5
- Cilengitide
Catalog No.:BCC3942
CAS No.:188968-51-6
- Melilotigenin C
Catalog No.:BCN1165
CAS No.:188970-21-0
- 1-(4-Hydroxy-2,2-dimethylchroman-6-yl)ethanone
Catalog No.:BCN7710
CAS No.:1890153-71-5
- Corynantheine
Catalog No.:BCN3746
CAS No.:18904-54-6
- NGB 2904
Catalog No.:BCC7435
CAS No.:189061-11-8
- [Ala92]-p16 (84-103)
Catalog No.:BCC5837
CAS No.:189064-08-2
Disruption of Nuclear Receptor Signaling Alters Triphenyl Phosphate-Induced Cardiotoxicity in Zebrafish Embryos.[Pubmed:29529285]
Toxicol Sci. 2018 May 1;163(1):307-318.
Triphenyl phosphate (TPHP) is an unsubstituted aryl phosphate ester used as a flame retardant and plasticizer within the United States. Using zebrafish as a model, the objectives of this study were to rely on (1) mRNA-sequencing to uncover pathways disrupted following embryonic TPHP exposure and (2) high-content screening to identify nuclear receptor ligands that enhance or mitigate TPHP-induced cardiotoxicity. Based on mRNA-sequencing, TPHP exposure from 24 to 72-h postfertilization (hpf) resulted in a concentration-dependent increase in the number of transcripts significantly affected at 72 hpf, and pathway analysis revealed that 5 out of 9 nuclear receptor pathways were associated with the retinoid X receptor (RXR). Based on a screen of 74 unique nuclear receptor ligands as well as follow-up experiments, 2 compounds-ciglitazone (a peroxisome proliferator-activated receptor gamma, or PPARgamma, agonist) and fenretinide (a pan-retinoic acid receptor, or RAR, agonist)-reliably mitigated TPHP-induced cardiotoxicity in the absence of effects on TPHP uptake or metabolism. As these data suggested that TPHP may be activating RXR (a heterodimer for both RARs and PPARgamma), we coexposed embryos to HX 531-a pan-RXR antagonist-from 24 to 72 hpf and, contrary to our hypothesis, found that coexposure to HX 531 significantly enhanced TPHP-induced cardiotoxicity. Using a luciferase reporter assay, we also found that TPHP did not activate nor inhibit chimeric human RXRalpha, RXRbeta, or RXRgamma, suggesting that TPHP does not directly bind nor interact with RXRs. Overall, our data suggest that TPHP may interfere with RXR-dependent pathways involved in cardiac development.
Docosahexaenoic acid induces adipose differentiation-related protein through activation of retinoid x receptor in human choriocarcinoma BeWo cells.[Pubmed:19571381]
Biol Pharm Bull. 2009 Jul;32(7):1177-82.
Adipose differentiation-related protein (ADRP) is associated with intracellular lipid droplets that accumulate neutral lipids. Here we report that ADRP expression in a human choriocarcinoma cell line, BeWo, is regulated through activation of retinoid X receptor (RXR) and peroxisome proliferator-activated receptor-gamma (PPARgamma). Incubation with docosahexaenoic acid (DHA) or oleic acid (OA) induced accumulation of triacylglycerol (TG) and ADRP in BeWo cells. DHA-induced ADRP expression was suppressed by RXR-antagonists, PA452 and HX531. However, oleic acid-induced ADRP expression was not blocked by the RXR-antagonists but by a PPARgamma-antagonist. Treatment of the cells with RXR-agonists, HX630 and PA024, increased Adrp transcripts, however, they alone did not change the levels of ADRP protein and TG in BeWo cells. Induction of ADRP protein was observed in the presence of a proteasome inhibitor, suggesting that ADRP is degraded under lipid-poor conditions. These results suggest that expression of ADRP is in part regulated by RXR and PPARgamma transcription factors, and DHA induces ADRP by acting as an endogenous agonist of RXR.
Vitamin A active metabolite, all-trans retinoic acid, induces spinal cord sensitization. II. Effects after intrathecal administration.[Pubmed:16847438]
Br J Pharmacol. 2006 Sep;149(1):65-72.
BACKGROUND AND PURPOSE: In our previous study (see accompanying paper) we observed that all-trans retinoic acid (ATRA) p.o. induces changes in spinal cord neuronal responses similar to those observed in inflammation-induced sensitization. In the present study we assessed the it. effects of ATRA, and its mechanisms of action. EXPERIMENTAL APPROACH: The effects of all drugs were studied after it. administration in nociceptive withdrawal reflexes using behavioural tests in awake male Wistar rats. KEY RESULTS: The administration of ATRA in normal rats induced a dose-dependent enhancement of nociceptive responses to noxious mechanical and thermal stimulation, as well as responses to innocuous stimulation. The intensity of the responses was similar to that observed in non-treated animals after carrageenan-induced inflammation. The effect induced by ATRA was fully prevented by the previous administration of the retinoic acid receptor (RAR) pan-antagonist LE540 but not by the retinoid X receptor (RXR) pan-antagonist HX531, suggesting a selective action on spinal cord RARs. The COX inhibitor dexketoprofen and the interleukin-1 receptor antagonist IL-1ra inhibited ATRA effect. The results indicate that COX and interleukin-1 are involved in the effects of ATRA in the spinal cord, similar to that seen in inflammation. CONCLUSIONS AND IMPLICATIONS: In conclusion, ATRA induces changes in the spinal cord similar to those observed in inflammation. The sensitization-like effect induced by ATRA was mediated by RARs and associated with a modulation of COX-2 and interleukin-1 activities. ATRA might be involved in the mechanisms underlying the initiation and/or maintenance of sensitization in the spinal cord.
Retinoid X receptor-antagonistic diazepinylbenzoic acids.[Pubmed:10748721]
Chem Pharm Bull (Tokyo). 1999 Dec;47(12):1778-86.
Several dibenzodiazepine derivatives were identified as novel retinoid X receptor (RXR) antagonists on the basis of inhibitory activity on retinoid-induced cell differentiation of human promyelocytic leukemia cells HL-60 and transactivation assay using retinoic acid receptors (RARs) and RXRs in COS-1 cells. 4-(5H-2,3-(2,5-Dimethyl-2,5-hexano)-5-n- propyldibenzo[b,e][1,4]diazepin-11-yl)benzoic acid (HX603, 6c) is an N-n-propyl derivative of an RXR pan-agonist HX600 (6a), and exhibited RXR-selective antagonistic activity. Similar RXR-antagonistic activities were observed with 4-(5H-2,3-(2,5-dimethyl-2,5-hexano)-5-methyl- 8-nitrodibenzo[b,e][1,4]diazepin-11-yl)benzoic acid (HX531, 7a) and 4-(5H-10,11-dihydro-5,10-dimethyl-2,3-(2,5-dimethyl- 2,5-hexano)-dibenzo[b,e][1,4]diazepin-11-yl)benzoic acid (HX711, 8b), which also inhibited transactivation of RARs induced by an RAR agonist, Am80. These compounds inhibited HL-60 cell differentiation induced by the combination of a low concentration of the retinoid agonist Am80 with an RXR agonist (a retinoid synergist, HX600). These results indicated that HX603 (6c), and the related RXR antagonists inhibit the activation of RAR-RXR heterodimers as well as RXR homodimers, which is a distinct characteristic different from that of the known RXR antagonist, LG100754 (9).


