L 760735High affinity NK1 receptor antagonist CAS# 188923-01-5 |
- BMS-708163 (Avagacestat)
Catalog No.:BCC2104
CAS No.:1146699-66-2
- DAPT (GSI-IX)
Catalog No.:BCC3618
CAS No.:208255-80-5
- YO-01027 (Dibenzazepine, DBZ)
Catalog No.:BCC2100
CAS No.:209984-56-5
- Semagacestat (LY450139)
Catalog No.:BCC3610
CAS No.:425386-60-3
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
- E 2012
Catalog No.:BCC1540
CAS No.:870843-42-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 188923-01-5 | SDF | Download SDF |
| PubChem ID | 9809076 | Appearance | Powder |
| Formula | C26H29ClF7N5O2 | M.Wt | 611.98 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 30 mM in water and to 50 mM in DMSO | ||
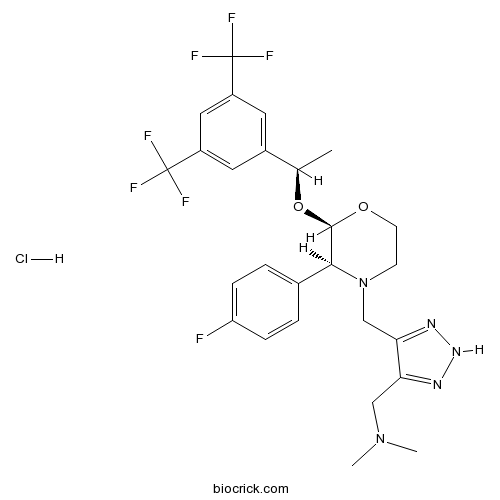
| Chemical Name | 1-[5-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)morpholin-4-yl]methyl]-2H-triazol-4-yl]-N,N-dimethylmethanamine;hydrochloride | ||
| SMILES | CC(C1=CC(=CC(=C1)C(F)(F)F)C(F)(F)F)OC2C(N(CCO2)CC3=NNN=C3CN(C)C)C4=CC=C(C=C4)F.Cl | ||
| Standard InChIKey | VZBKOBSSEVXFNF-QIRDZIKRSA-N | ||
| Standard InChI | InChI=1S/C26H28F7N5O2.ClH/c1-15(17-10-18(25(28,29)30)12-19(11-17)26(31,32)33)40-24-23(16-4-6-20(27)7-5-16)38(8-9-39-24)14-22-21(13-37(2)3)34-36-35-22;/h4-7,10-12,15,23-24H,8-9,13-14H2,1-3H3,(H,34,35,36);1H/t15-,23+,24-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity NK1 receptor antagonist (IC50 = 0.19 nM at human NK1 receptors). Selective (>300-fold) over h-NK2 and h-NK3 receptors. Exhibits anxiolytic and antidepressant-like effects. Orally active. |

L 760735 Dilution Calculator

L 760735 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.634 mL | 8.1702 mL | 16.3404 mL | 32.6808 mL | 40.851 mL |
| 5 mM | 0.3268 mL | 1.634 mL | 3.2681 mL | 6.5362 mL | 8.1702 mL |
| 10 mM | 0.1634 mL | 0.817 mL | 1.634 mL | 3.2681 mL | 4.0851 mL |
| 50 mM | 0.0327 mL | 0.1634 mL | 0.3268 mL | 0.6536 mL | 0.817 mL |
| 100 mM | 0.0163 mL | 0.0817 mL | 0.1634 mL | 0.3268 mL | 0.4085 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Junipediol B 8-O-glucoside
Catalog No.:BCN4022
CAS No.:188894-19-1
- Methyl tanshinonate
Catalog No.:BCN2553
CAS No.:18887-19-9
- Hydroxytanshinone IIA
Catalog No.:BCN2497
CAS No.:18887-18-8
- DMA
Catalog No.:BCC1532
CAS No.:188860-26-6
- HX 630
Catalog No.:BCC6083
CAS No.:188844-52-2
- HX 531
Catalog No.:BCC6082
CAS No.:188844-34-0
- Streptozotocin
Catalog No.:BCN3834
CAS No.:18883-66-4
- 8-Glucosyl-5,7-dihydroxy-2-(1-methylpropyl)chromone
Catalog No.:BCN7505
CAS No.:188818-27-1
- SC 560
Catalog No.:BCC7111
CAS No.:188817-13-2
- N-Acetylcaprolactam
Catalog No.:BCC9081
CAS No.:1888-91-1
- 4,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6492
CAS No.:188742-80-5
- 3-hydroxymorindone
Catalog No.:BCN3126
CAS No.:80368-74-7
- Cilengitide
Catalog No.:BCC3942
CAS No.:188968-51-6
- Melilotigenin C
Catalog No.:BCN1165
CAS No.:188970-21-0
- 1-(4-Hydroxy-2,2-dimethylchroman-6-yl)ethanone
Catalog No.:BCN7710
CAS No.:1890153-71-5
- Corynantheine
Catalog No.:BCN3746
CAS No.:18904-54-6
- NGB 2904
Catalog No.:BCC7435
CAS No.:189061-11-8
- [Ala92]-p16 (84-103)
Catalog No.:BCC5837
CAS No.:189064-08-2
- Oroselol
Catalog No.:BCN3907
CAS No.:1891-25-4
- Hulupinic acid
Catalog No.:BCN8019
CAS No.:1891-42-5
- Salmefamol
Catalog No.:BCC1919
CAS No.:18910-65-1
- Bruceantinoside C
Catalog No.:BCN1166
CAS No.:112899-35-1
- Fas C- Terminal Tripeptide
Catalog No.:BCC1019
CAS No.:189109-90-8
- 3'-O-Methylmurraol
Catalog No.:BCN7471
CAS No.:1891097-17-8
The substance P (NK1) receptor antagonist L-760735 inhibits fear conditioning in gerbils.[Pubmed:12646288]
Neuropharmacology. 2003 Mar;44(4):516-23.
The ability of the substance P (NK(1) receptor) antagonist (SPA) L-760735 to inhibit conditioned fear was assessed in gerbils using a four plate apparatus. Animals that had been treated with diazepam (3 mg/kg) or L-760735 (3 mg/kg) 30 min before a 3 min conditioning session in the apparatus exhibited a release of plate crossings during the retest session approximately 3 h later. Plate crossings were also increased when animals received diazepam or L-760735 30 min before the retest session. In contrast, fluoxetine and venlafaxine (30 mg/kg) did not exhibit anxiolytic-like effects. During the retest session, gerbils drummed their hind feet on the floor; this behaviour was not observed spontaneously in gerbils that were naive to the apparatus. Foot drumming was abolished by pretreatment with L-760735 or diazepam (3 mg/kg) but was markedly increased following administration of fluoxetine or venlafaxine (30 mg/kg). Foot drumming elicited by aversive conditioning alone or in combination with fluoxetine was abolished by administration of L-760735 and by amygdala lesions involving the basolateral and lateral nuclei, indicating that this behaviour is an alarm signal or fear response mediated via release of substance P in brain circuits involving the amygdala. The observations provide further evidence for an anxiolytic-like profile of SPAs in preclinical assays and demonstrate a clear difference between the actions of SPAs and established antidepressant drugs.
Chronic psychosocial stress in tree shrews: effect of the substance P (NK1 receptor) antagonist L-760735 and clomipramine on endocrine and behavioral parameters.[Pubmed:15875166]
Psychopharmacology (Berl). 2005 Sep;181(2):207-16.
RATIONALE: Substance P and its preferred receptor, the neurokinin 1 receptor (NK(1)R), have been proposed as possible targets for new antidepressant therapies, although results of a recently completed phase III trial failed to demonstrate that the NK(1)R antagonist MK-869 is more effective than placebo in the treatment of depression. METHODS: In the present study, we compared the effects of the NK(1)R antagonist L-760735 with the tricyclic antidepressant clomipramine on endocrine and behavioral parameters in chronically stressed tree shrews. Animals were subjected to a 7-day period of psychosocial stress before receiving daily oral administration of L-760735 (10 mg/kg/day) or clomipramine (50 mg/kg/day). The psychosocial stress continued throughout the treatment period of 21 days. Daily morning urine was collected to measure cortisol and norepinephrine levels. All animals were videotaped daily and three types of behavior were analyzed. RESULTS: Chronic psychosocial stress resulted in a significant increase of urinary cortisol and norepinephrine concentrations. Moreover, stressed animals displayed decreased marking behavior and locomotor activity, while grooming remained unaffected. Neither treatment with clomipramine nor L-760735 was able to normalize the stress-induced elevation of cortisol or norepinephrine. On the behavioral parameters, L-760735 had a time-dependent restorative influence on marking behavior close to normal levels, without affecting locomotor activity. Grooming behavior was significantly increased by the 3 weeks of drug treatment. CONCLUSIONS: These results suggest that L-760735 was able to counteract certain stress-induced behavioral alterations in an animal model of depression.
Anxiolytic actions of the substance P (NK1) receptor antagonist L-760735 and the 5-HT1A agonist 8-OH-DPAT in the social interaction test in gerbils.[Pubmed:11595206]
Brain Res. 2001 Oct 12;915(2):170-5.
The gerbil social interaction test has previously detected anxiolytic effects of nicotine and diazepam. In the present study, the high affinity substance P (NK(1)) receptor antagonist L-760735 (3 mg/kg) significantly increased the time spent in social interaction, whereas its low affinity analogue L-781773 (3 mg/kg) was without effect. Diazepam (0.1 mg/kg) and the 5-HT(1A) receptor agonist 8-OH-DPAT (0.003 and 0.01 mg/kg) also increased social interaction, whereas an acute dose of the selective serotonin re-uptake inhibitor fluoxetine (10 mg/kg) decreased the time spent in social interaction. Diazepam (0.1 mg/kg) significantly increased locomotor activity, but this effect was independent of the increase in social interaction. The other drugs tested were without effect on locomotor activity. The present findings suggest that the gerbil social interaction may well provide a useful assay for detecting both anxiolytic and anxiogenic compounds, and suggests that the high affinity NK(1) receptor antagonist L-760735 may prove to be useful as an anxiolytic therapy.
Intra-amygdala injection of the substance P [NK(1) receptor] antagonist L-760735 inhibits neonatal vocalisations in guinea-pigs.[Pubmed:11445193]
Neuropharmacology. 2001 Jul;41(1):130-7.
The involvement of the basolateral amygdala in mediating the inhibition of neonatal vocalisation by substance P (NK(1) receptor) antagonists was examined. These studies determined whether the time course for separation-induced vocalisations in guinea-pig pups coincided with NK(1) receptor internalisation (a marker of substance P release) in the amygdala, and whether vocalisations could be blocked by focal injection of the NK(1) receptor antagonist L-760735 into this brain region. The peak period for neonatal vocalisations occurred 5-10 min following maternal separation. This coincided with the peak increase in the number of cells in the basolateral amygdala exhibiting NK(1) receptor endocytosis, consistent with the proposal that substance P is released in the amygdala as a result of isolation stress. Focal injection of L-760735 (15 nmol per side) but not L-770765 (an analogue of L-760735 which has low NK(1) receptor affinity) into the basolateral amygdala attenuated separation-induced vocalisations. In contrast, injection of L-760735 (15 nmol per side) into the dorsal ventricular nucleus of the thalamus, a region with relatively low density of NK(1) receptors, had no effect on neonatal vocalisations. These findings are consistent with other evidence that the amygdala is one possible site of action for the inhibition of neonatal vocalisations by substance P antagonists.
Correlation of neurokinin (NK) 1 receptor occupancy in gerbil striatum with behavioral effects of NK1 antagonists.[Pubmed:11961054]
J Pharmacol Exp Ther. 2002 May;301(2):536-42.
Interest in central neurokinin (NK) 1 receptors has increased based on reports of the therapeutic potential for NK1 antagonists in anxiety and depression. In these studies, an ex vivo binding procedure was used to correlate NK1 receptor occupancy in striatum by NK1 antagonists with their potency to inhibit NK1 agonist-induced foot tapping in gerbils (GFT). The following compounds were administered orally: CP-99,994 [(+)-cis-n-[(2-methoxyphenyl)methyl]-2-phenyl-3-piperidinamine), L-742,694 [5-[[2(S)-[[3,5-bis(trifluoromethyl)phenyl]methoxy]-3(S)-phenyl-4-morpholinyl]met hyl]-2,4-dihydro-3H-1,2,4-triazol-3-one]), MK-869 [5-[[2(R)-[1(R)-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3(S)-(4-fluorophenyl)-4-m orpholinyl]methyl]-2,4-dihydro-3H-1,2,4-triazol-3-one], CP-122,721 [cis-n-[[2-methoxy-5-(trifluoromethoxy)phenyl]methyl]-2-phenyl-3-piperidinamine], L-760,735-F [4-[[2(R)-[1(R)-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3(S)-(4-fluorophenyl)-4-m orpholinyl]methyl]-N,N-dimethyl-1H-1,2,3-triazole-5- methanamine], GR205171 [N-[[2-methoxy-5-[5-(trifluoromethyl)-1H-tetrazol-1-yl]phenyl]methy]-2(S)-phenyl- 3(S)-piperidinamine], L-733,060 [(2S,3S)3-([3,5-bis(trifluoro methyl)phenyl]methoxy)-2-phenylpiperidine], and L-733,061 [(2R,3R)-3-([3,5-bis(trifluoromethyl)phenyl]methoxy)-2-phenylpiperidine]. Two hours later, gerbils received the NK1 agonist GR73632 [H(2)N-(CH(2))(4)-CO-Phe-Pro-NMe-Leu-Met-NH(2)] i.c.v. and foot tapping was measured for 5 min. The same procedure was used for ex vivo binding studies except that saline, rather than agonist, was administered i.c.v. before dissection of the striatum. The tissue homogenate was then used in an equilibrium radioligand binding assay. When IC(50) values for inhibition of ex vivo (125)I-substance P binding by NK1 antagonists were compared with the corresponding EC(50) values for inhibition of GFT, a significant positive correlation was observed (r(2) = 0.97, p < 0.001). This result indicates that increased NK1 receptor occupancy in striatum by NK1 antagonists parallels the inhibition of agonist-mediated GFT. For all compounds, the dose that produced the maximum inhibition of GFT resulted in less than 100% ex vivo receptor occupancy in striatum. When gerbils did not receive the i.c.v. saline injection before ex vivo binding, thereby leaving the blood-brain barrier (BBB) intact, the IC(50) values for antagonists were unchanged, suggesting that potential damage to the BBB caused by the i.c.v. injection did not affect determinations of antagonist potency in the GFT model.
An orally active, water-soluble neurokinin-1 receptor antagonist suitable for both intravenous and oral clinical administration.[Pubmed:11708932]
J Med Chem. 2001 Nov 22;44(24):4296-9.
1-(5-[[(2R,3S)-2-([(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethyl]oxy)-3-(4-fluorop henyl)morpholin-4-yl]methyl]-2H-1,2,3-triazol-4-yl)-N,N-dimethylmethanamine hydrochloride 3 is a high affinity, orally active, h-NK(1) receptor antagonist with a long central duration of action and a solubility in water of >100 mg/mL. The construction of the 5-dimethylaminomethyl 1,2,3-triazol-4-yl unit, which incorporates the solubilizing group of 3, was accomplished by thermal rearrangement of a propargylic azide in the presence of dimethylamine. Compound 3 is highly effective in pre-clinical tests that are relevant to clinical efficacy in emesis and depression.


